Release of Cathepsin B in Cytosol Causes Cell Death in Acute Pancreatitis
- PMID: 27519471
- PMCID: PMC5037034
- DOI: 10.1053/j.gastro.2016.06.042
Release of Cathepsin B in Cytosol Causes Cell Death in Acute Pancreatitis
Abstract
Background & aims: Experimental studies in acute pancreatitis (AP) suggest a strong association of acinar cell injury with cathepsin B-dependent intracellular activation of trypsin. However, the molecular events subsequent to trypsin activation and their role, if any, in cell death is not clear. In this study, we have explored intra-acinar events downstream of trypsin activation that lead to acinar cell death.
Methods: Acinar cells prepared from the pancreas of rats or mice (wild-type, trypsinogen 7, or cathepsin B-deleted) were stimulated with supramaximal cerulein, and the cytosolic activity of cathepsin B and trypsin was evaluated. Permeabilized acini were used to understand the differential role of cytosolic trypsin vs cytosolic cathepsin B in activation of apoptosis. Cell death was evaluated by measuring specific markers for apoptosis and necrosis.
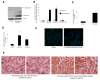
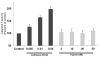
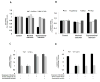
Results: Both in vitro and in vivo studies have suggested that during AP cathepsin B leaks into the cytosol from co-localized organelles, through a mechanism dependent on active trypsin. Cytosolic cathepsin B but not trypsin activates the intrinsic pathway of apoptosis through cleavage of bid and activation of bax. Finally, excessive release of cathepsin B into the cytosol can lead to cell death through necrosis.
Conclusions: This report defines the role of trypsin in AP and shows that cytosolic cathepsin B but not trypsin activates cell death pathways. This report also suggests that trypsin is a requisite for AP only because it causes release of cathepsin B into the cytosol.
Keywords: Apoptosis; Bid Cleavage; Cytosolic Cathepsin B; Necrosis.
Copyright © 2016. Published by Elsevier Inc.
Figures




References
-
- Chiari H. ÜberdieSelbstverdauung des menschlichenPankreas. Zeitschriftfür Heilkunde. 1896;17:69–96.
-
- Grady T, Saluja A, Kaiser A, et al. Edema and intrapancreatic trypsinogen activation precede glutathione depletion during caerulein pancreatitis. Am J Physiol. 1996;271:G20–6. - PubMed
-
- Saluja A, Hashimoto S, Saluja M, et al. Subcellular redistribution of lysosomal enzymes during caerulein-induced pancreatitis. Am J Physiol. 1987;253:G508–16. - PubMed
-
- Watanabe O, Baccino FM, Steer ML, et al. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984;246:G457–67. - PubMed
-
- Saluja AK, Donovan EA, Yamanaka K, et al. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113:304–10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

