New method of preoxygenation for orotracheal intubation in patients with hypoxaemic acute respiratory failure in the intensive care unit, non-invasive ventilation combined with apnoeic oxygenation by high flow nasal oxygen: the randomised OPTINIV study protocol
- PMID: 27519921
- PMCID: PMC4985915
- DOI: 10.1136/bmjopen-2016-011298
New method of preoxygenation for orotracheal intubation in patients with hypoxaemic acute respiratory failure in the intensive care unit, non-invasive ventilation combined with apnoeic oxygenation by high flow nasal oxygen: the randomised OPTINIV study protocol
Abstract
Introduction: Tracheal intubation in the intensive care unit (ICU) is associated with severe life-threatening complications including severe hypoxaemia. Preoxygenation before intubation has been recommended in order to decrease such complications. Non-invasive ventilation (NIV)-assisted preoxygenation allows increased oxygen saturation during the intubation procedure, by applying a positive end-expiratory pressure (PEEP) to prevent alveolar derecruitment. However, the NIV mask has to be taken off after preoxygenation to allow the passage of the tube through the mouth. The patient with hypoxaemia does not receive oxygen during this period, at risk of major hypoxaemia. High-flow nasal cannula oxygen therapy (HFNC) has a potential for apnoeic oxygenation during the apnoea period following the preoxygenation with NIV. Whether application of HFNC combined with NIV is more effective at reducing oxygen desaturation during the intubation procedure compared with NIV alone for preoxygenation in patients with hypoxaemia in the ICU with acute respiratory failure remains to be established.
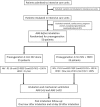
Methods and analysis: The HFNC combined to NIV for decreasing oxygen desaturation during the intubation procedure in patients with hypoxaemia in the ICU (OPTINIV) trial is an investigator-initiated monocentre randomised controlled two-arm trial with assessor-blinded outcome assessment. The OPTINIV trial randomises 50 patients with hypoxaemia requiring orotracheal intubation for acute respiratory failure to receive NIV (pressure support=10, PEEP=5, fractional inspired oxygen (FiO2)=100%) combined with HFNC (flow=60 L/min, FiO2=100%, interventional group) or NIV alone (reference group) for preoxygenation. The primary outcome is lowest oxygen saturation during the intubation procedure. Secondary outcomes are intubation-related complications, quality of preoxygenation and ICU mortality.
Ethics and dissemination: The study project has been approved by the appropriate ethics committee (CPP Sud-Méditerranée). Informed consent is required. If combined application of HFNC and NIV for preoxygenation of patients with hypoxaemia in the ICU proves superior to NIV preoxygenation, its use will become standard practice, thereby decreasing hypoxaemia during the intubation procedure and potential complications related to intubation.
Trial registration number: NCT02530957.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures


Similar articles
-
Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial.Intensive Care Med. 2016 Dec;42(12):1877-1887. doi: 10.1007/s00134-016-4588-9. Epub 2016 Oct 11. Intensive Care Med. 2016. PMID: 27730283 Clinical Trial.
-
Preoxygenation with non-invasive ventilation versus high-flow nasal cannula oxygen therapy for intubation of patients with acute hypoxaemic respiratory failure in ICU: the prospective randomised controlled FLORALI-2 study protocol.BMJ Open. 2017 Dec 22;7(12):e018611. doi: 10.1136/bmjopen-2017-018611. BMJ Open. 2017. PMID: 29275345 Free PMC article.
-
Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial.Lancet Respir Med. 2019 Apr;7(4):303-312. doi: 10.1016/S2213-2600(19)30048-7. Epub 2019 Mar 18. Lancet Respir Med. 2019. PMID: 30898520 Clinical Trial.
-
High flow nasal cannula oxygen versus noninvasive ventilation in adult acute respiratory failure: a systematic review of randomized-controlled trials.Eur J Emerg Med. 2019 Feb;26(1):9-18. doi: 10.1097/MEJ.0000000000000557. Eur J Emerg Med. 2019. PMID: 29923842
-
Preoxygenation before intubation in adult patients with acute hypoxemic respiratory failure: a network meta-analysis of randomized trials.Crit Care. 2019 Sep 18;23(1):319. doi: 10.1186/s13054-019-2596-1. Crit Care. 2019. PMID: 31533792 Free PMC article.
Cited by
-
Effect of cold environments on technical performance and perceived workload and stress during advanced medical procedures: a randomized controlled simulation study.Scand J Trauma Resusc Emerg Med. 2025 Jul 1;33(1):113. doi: 10.1186/s13049-025-01373-8. Scand J Trauma Resusc Emerg Med. 2025. PMID: 40598316 Free PMC article. Clinical Trial.
-
Effects of Preoxygenation with Tidal Volume Breathing Followed by Apneic Oxygenation with and without Continuous Positive Airway Pressure on Duration of Safe Apnea Time and Arterial Blood Gases.Anesth Essays Res. 2018 Jan-Mar;12(1):229-233. doi: 10.4103/aer.AER_219_17. Anesth Essays Res. 2018. PMID: 29628587 Free PMC article.
-
Comparison of Arterial Oxygenation and Acid-Base Balance with the use of Transnasal Humidified Rapid-insufflation Ventilatory Exchange versus Tidal Volume Breathing with Continuous Positive Airway Pressure for Preoxygenation and Apneic Ventilation.Anesth Essays Res. 2018 Jan-Mar;12(1):246-250. doi: 10.4103/aer.AER_13_18. Anesth Essays Res. 2018. PMID: 29628590 Free PMC article.
-
Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial.Intensive Care Med. 2016 Dec;42(12):1877-1887. doi: 10.1007/s00134-016-4588-9. Epub 2016 Oct 11. Intensive Care Med. 2016. PMID: 27730283 Clinical Trial.
-
Oxygen therapy in the intensive care unit.Med Gas Res. 2025 Dec 1;15(4):478-487. doi: 10.4103/mgr.MEDGASRES-D-24-00143. Epub 2025 Apr 29. Med Gas Res. 2025. PMID: 40300883 Free PMC article. Review.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
