False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis: an empirical review
- PMID: 27519923
- PMCID: PMC4985805
- DOI: 10.1136/bmjopen-2016-011890
False-positive findings in Cochrane meta-analyses with and without application of trial sequential analysis: an empirical review
Abstract
Objective: Many published meta-analyses are underpowered. We explored the role of trial sequential analysis (TSA) in assessing the reliability of conclusions in underpowered meta-analyses.
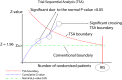
Methods: We screened The Cochrane Database of Systematic Reviews and selected 100 meta-analyses with a binary outcome, a negative result and sufficient power. We defined a negative result as one where the 95% CI for the effect included 1.00, a positive result as one where the 95% CI did not include 1.00, and sufficient power as the required information size for 80% power, 5% type 1 error, relative risk reduction of 10% or number needed to treat of 100, and control event proportion and heterogeneity taken from the included studies. We re-conducted the meta-analyses, using conventional cumulative techniques, to measure how many false positives would have occurred if these meta-analyses had been updated after each new trial. For each false positive, we performed TSA, using three different approaches.
Results: We screened 4736 systematic reviews to find 100 meta-analyses that fulfilled our inclusion criteria. Using conventional cumulative meta-analysis, false positives were present in seven of the meta-analyses (7%, 95% CI 3% to 14%), occurring more than once in three. The total number of false positives was 14 and TSA prevented 13 of these (93%, 95% CI 68% to 98%). In a post hoc analysis, we found that Cochrane meta-analyses that are negative are 1.67 times more likely to be updated (95% CI 0.92 to 2.68) than those that are positive.
Conclusions: We found false positives in 7% (95% CI 3% to 14%) of the included meta-analyses. Owing to limitations of external validity and to the decreased likelihood of updating positive meta-analyses, the true proportion of false positives in meta-analysis is probably higher. TSA prevented 93% of the false positives (95% CI 68% to 98%).
Keywords: EPIDEMIOLOGY; PUBLIC HEALTH; STATISTICS & RESEARCH METHODS.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials