Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia
- PMID: 27520294
- PMCID: PMC5436271
- DOI: 10.1158/2159-8290.CD-16-0313
Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia
Abstract
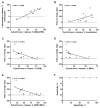
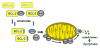
We present a phase II, single-arm study evaluating 800 mg daily venetoclax, a highly selective, oral small-molecule B-cell leukemia/lymphoma-2 (BCL2) inhibitor in patients with high-risk relapsed/refractory acute myelogenous leukemia (AML) or unfit for intensive chemotherapy. Responses were evaluated following revised International Working Group (IWG) criteria. The overall response rate was 19%; an additional 19% of patients demonstrated antileukemic activity not meeting IWG criteria (partial bone marrow response and incomplete hematologic recovery). Twelve (38%) patients had isocitrate dehydrogenase 1/2 mutations, of whom 4 (33%) achieved complete response or complete response with incomplete blood count recovery. Six (19%) patients had BCL2-sensitive protein index at screening, which correlated with time on study. BH3 profiling was consistent with on-target BCL2 inhibition and identified potential resistance mechanisms. Common adverse events included nausea, diarrhea and vomiting (all grades), and febrile neutropenia and hypokalemia (grade 3/4). Venetoclax demonstrated activity and acceptable tolerability in patients with AML and adverse features.
Significance: Venetoclax monotherapy demonstrated clinical activity in patients with AML (relapsed/refractory or unfit for intensive chemotherapy) with a tolerable safety profile in this phase II study. Predictive markers of response consistent with BCL2 dependence were identified. Clinical and preclinical findings provide a compelling rationale to evaluate venetoclax combined with other agents in AML. Cancer Discov; 6(10); 1106-17. ©2016 AACRSee related commentary by Pullarkat and Newman, p. 1082This article is highlighted in the In This Issue feature, p. 1069.
©2016 American Association for Cancer Research.
Conflict of interest statement
M. Konopleva has been a consultant to and received research funding from AbbVie and Genentech. D.A. Pollyea is an advisory board member for Agios, Ariad, Celgene, Karyopharm, and Pfizer. L. Hogdal has nothing to disclose. W. Blum has been a consultant to Genentech. C. DiNardo has received research funding from Abbvie, Agios, Celgene, CTI, Daiichi-Sankyo, and Novartis. T. Kadia has received research funding from BMS and Celgene, and has been a consultant to Ariad and Novartis. R. Stone has been a consultant to AbbVie, Agios, Amgen, Arrog, Celator, Celgene, Janssen, Karyopharm, Merck, Novartis, Pfizer, Genentech, and Sunesis. H. Kantarjian has received research funding from Ariad, BMS, Novartis, and Pfizer. A. Letai has been an advisor to and his laboratory has received research funds from AbbVie, AstraZeneca, Tetralogic, and XrX. J. Potluri, B. Chyla, T. Busman, E. McKeegan, A.H. Salem, M. Zhu, J.L. Ricker, M. Dunbar, R. Kirby, N. Falotico, J. Leverson, R. Humerickhouse, and M. Mabry are AbbVie employees and may own stock.
Figures

Comment in
-
BCL2 Inhibition by Venetoclax: Targeting the Achilles' Heel of the Acute Myeloid Leukemia Stem Cell?Cancer Discov. 2016 Oct;6(10):1082-1083. doi: 10.1158/2159-8290.CD-16-0921. Cancer Discov. 2016. PMID: 27698099
References
-
- Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–8. - PubMed
-
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–65. - PubMed
-
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

