Triterpenoids from Acacia ataxacantha DC: antimicrobial and antioxidant activities
- PMID: 27520306
- PMCID: PMC4983094
- DOI: 10.1186/s12906-016-1266-y
Triterpenoids from Acacia ataxacantha DC: antimicrobial and antioxidant activities
Abstract
Background: Acacia ataxacantha is a medicinal specie used extensively in traditional medicine of Benin republic to treat infectious diseases. Our previous study showed interesting antibacterial and antifungal activities against six strains of bacteria and six strains of fungi. The aim of this study was to investigate the antimicrobial and antioxidant activities of compounds isolated from A. ataxacantha.
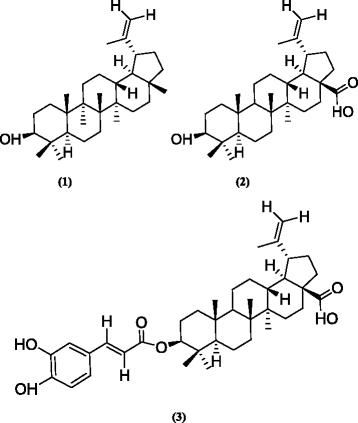
Methods: Chromatographic and spectroscopic methods were used to isolate and identify three compounds (1-3) from the bark of A. ataxacantha. Phytochemical investigation of A. ataxacantha (Fabaceae) led to the isolation of three triterpenoids (1-3). The structure of isolated compounds was established by differents spectroscopic methods such as UV, (1)H NMR, (13)C NMR, 2D NMR and Mass. All isolated compounds were tested for antimicrobial activity using agar disc-diffusion and microdilution methods. The radical scavenging activity of isolated compounds was assessed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) method.
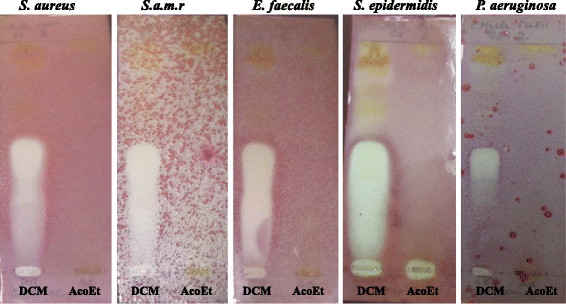
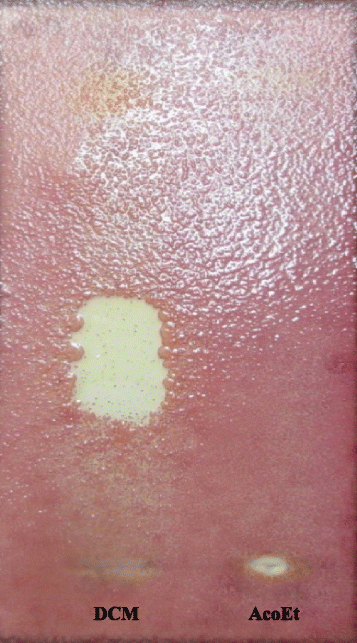
Results: Phytochemical investigation led to the isolation and identification of lupeol (1), betulinic acid (2) and betulinic acid-3-trans-caffeate (3). Moderate antimicrobial activity was obtained with compound 3 against methicillin-resitant Staphylococcus aureus, Enterococcus feacalis and Pseudomonas aeruginosa with MIC value of 25 μg/ml and Staphylococcus aureus (MIC of 50 μg/ml). Compounds 3 was more active against Staphylococcus epidermidis and Candida albicans with a MIC value of 12.5 μg/ml in boths cases. Compounds 3 had also interesting antioxidant activity with an IC50 of 3.57 μg/ml compared to quercetin (1.04 μg/ml).
Conclusion: The overall results of this study provide evidence that the compound 3, isolated from A. ataxacantha, exhibit antimicrobial activity against Gram-positive and Gram-negative bacteria and yeast, especially against C. albicans.
Keywords: Acacia ataxacantha; Antibacterial; Antifungal; Antioxidant; Betulinic acid-3-trans-caffeate; Triterpene.
Figures
References
-
- Lalitha P, Sripathi SK, Jayanthi P. Acute toxicity study of extracts of Eichhornia Crassipes (Mart.) Solms. Asian J Pharm Clin Res. 2012;5(4):59–61.
-
- Adjanohoun I, Ahyi M, Aké A, Akouegninou A, Dalmeida J, Akpovo F, Bouke FK, et al. Contribution aux études ethnobotaniques et floristiques en République Populaire du Bénin. Paris: ACCA; 1989. p. 852.
-
- MacDonald I, Joseph OE, Harriet ME. Documentation of medicinal plants sold in markets in Abeokuta, Nigeria. Trop J Pharm Res. 2010;9(2):110–118.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

