Quantifying the removal of red blood cells in Macaca mulatta during a Plasmodium coatneyi infection
- PMID: 27520455
- PMCID: PMC4983012
- DOI: 10.1186/s12936-016-1465-5
Quantifying the removal of red blood cells in Macaca mulatta during a Plasmodium coatneyi infection
Abstract
Background: Malaria is the most deadly parasitic disease in humans globally, and the long-time coexistence with malaria has left indelible marks in the human genome that are the causes of a variety of genetic disorders. Although anaemia is a common clinical complication of malaria, the root causes and mechanisms involved in the pathogenesis of malarial anaemia are unclear and difficult to study in humans. Non-human primate (NHP) model systems enable the mechanistic study and quantification of underlying causative factors of malarial anaemia, and particularly the onset of severe anaemia.
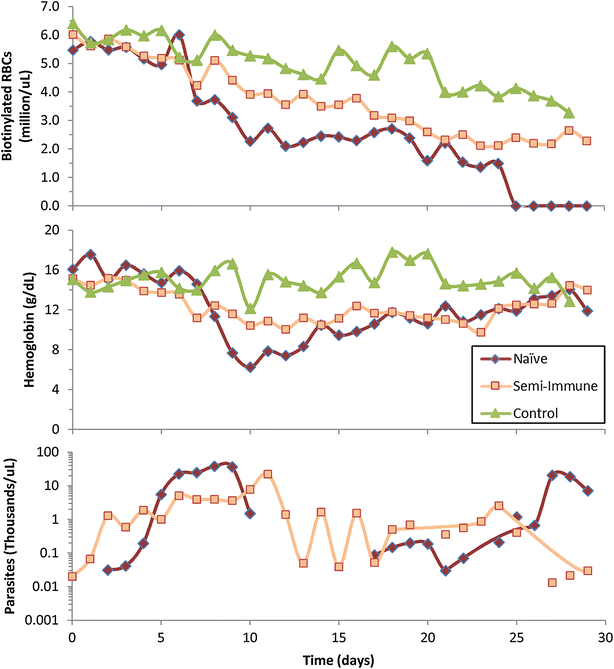
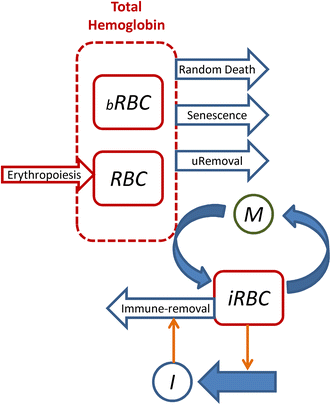
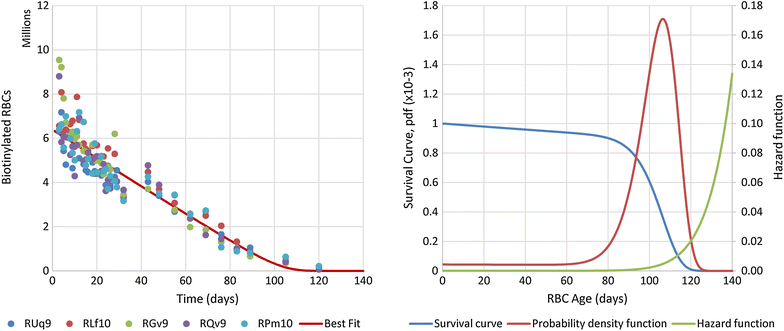
Methods: Data were obtained in the course of Plasmodium coatneyi infections of malaria-naïve and semi-immune rhesus macaques (Macaca mulatta), whose red blood cells (RBCs) were labelled in situ with biotin at the time the infections were initiated. The data were used for a survival analysis that permitted, for the first time, an accurate estimation of the lifespan of erythrocytes in macaques. The data furthermore formed the basis for the development and parameterization of a recursive dynamic model of erythrocyte turnover, which was used for the quantification of RBC production and removal in each macaque.
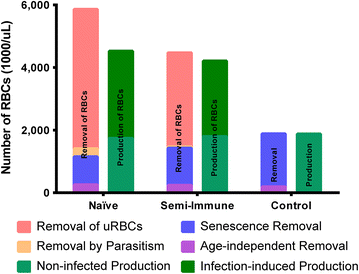
Results: The computational analysis demonstrated that the lifespan of erythrocytes in macaques is 98 ± 21 days. The model also unambiguously showed that death due to senescence and parasitaemia is not sufficient to account for the extent of infection-induced anaemia. Specifically, the model permits, for the first time, the quantification of the different causes of RBC death, namely, normal senescence, age-independent random loss, parasitization, and bystander effects in uninfected cells. Such a dissection of the overall RBC removal process is hardly possible with experimental means alone. In the infected malaria-naïve macaques, death of erythrocytes by normal physiological senescence processes accounts for 20 % and parasitization for only 4 %, whereas bystander effects are associated with an astonishing 76 % of total RBC losses. Model-based comparisons of alternative mechanisms involved in the bystander effect revealed that most of the losses are likely due to a process of removing uninfected RBCs of all age classes and only minimally due to an increased rate of senescence of the uninfected RBCs.
Conclusions: A new malaria blood-stage model was developed for the analysis of data characterizing P. coatneyi infections of M. mulatta. The model used a discrete and recursive framework with age-structure that allowed the quantification of the most significant pathophysiological processes of RBC removal. The computational results revealed that the malarial anaemia caused by this parasite is mostly due to a loss of uninfected RBCs by an age-independent process. The biological identity and complete mechanism of this process is not fully understood and requires further investigation.
Keywords: Macaca mulatta; Malarial anaemia; Mathematical model; Plasmodium coatneyi; Red blood cell removal.
Figures
Similar articles
-
Analysis of erythrocyte dynamics in Rhesus macaque monkeys during infection with Plasmodium cynomolgi.Malar J. 2018 Nov 6;17(1):410. doi: 10.1186/s12936-018-2560-6. Malar J. 2018. PMID: 30400896 Free PMC article.
-
A primate model of severe malarial anaemia: a comparative pathogenesis study.Sci Rep. 2019 Dec 12;9(1):18965. doi: 10.1038/s41598-019-55377-3. Sci Rep. 2019. PMID: 31831787 Free PMC article.
-
Plasmodium cynomolgi infections in rhesus macaques display clinical and parasitological features pertinent to modelling vivax malaria pathology and relapse infections.Malar J. 2016 Sep 2;15(1):451. doi: 10.1186/s12936-016-1480-6. Malar J. 2016. PMID: 27590312 Free PMC article.
-
A Review of Plasmodium coatneyi-Macaque Models of Severe Malaria.Vet Pathol. 2015 Nov;52(6):998-1011. doi: 10.1177/0300985815583098. Epub 2015 Jun 15. Vet Pathol. 2015. PMID: 26077782 Review.
-
Systems biology of malaria explored with nonhuman primates.Malar J. 2022 Jun 7;21(1):177. doi: 10.1186/s12936-022-04199-2. Malar J. 2022. PMID: 35672852 Free PMC article. Review.
Cited by
-
Case Report: Severe and Complicated Cynomolgi Malaria in a Rhesus Macaque Resulted in Similar Histopathological Changes as Those Seen in Human Malaria.Am J Trop Med Hyg. 2017 Aug;97(2):548-555. doi: 10.4269/ajtmh.16-0742. Am J Trop Med Hyg. 2017. PMID: 28829738 Free PMC article.
-
Humoral immunity prevents clinical malaria during Plasmodium relapses without eliminating gametocytes.PLoS Pathog. 2019 Sep 19;15(9):e1007974. doi: 10.1371/journal.ppat.1007974. eCollection 2019 Sep. PLoS Pathog. 2019. PMID: 31536608 Free PMC article.
-
Clinical recovery of Macaca fascicularis infected with Plasmodium knowlesi.Malar J. 2021 Dec 30;20(1):486. doi: 10.1186/s12936-021-03925-6. Malar J. 2021. PMID: 34969401 Free PMC article.
-
ZOOMICS: Comparative Metabolomics of Red Blood Cells From Guinea Pigs, Humans, and Non-human Primates During Refrigerated Storage for Up to 42 Days.Front Physiol. 2022 Mar 21;13:845347. doi: 10.3389/fphys.2022.845347. eCollection 2022. Front Physiol. 2022. PMID: 35388289 Free PMC article.
-
Insights into malaria pathogenesis gained from host metabolomics.PLoS Pathog. 2020 Nov 12;16(11):e1008930. doi: 10.1371/journal.ppat.1008930. eCollection 2020 Nov. PLoS Pathog. 2020. PMID: 33180883 Free PMC article. No abstract available.
References
-
- WHO. World Malaria Report 2014. Geneva: World Health Organization; 2014. http://www.who.int/malaria/publications/world_malaria_report_2014/en/.
-
- Nagel RL, Roth EF., Jr Malaria and red cell genetic defects. Blood. 1989;74:1213–1221. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

