Safety of atorvastatin in Asian patients within clinical trials
- PMID: 27520479
- PMCID: PMC5129583
- DOI: 10.1111/1755-5922.12214
Safety of atorvastatin in Asian patients within clinical trials
Abstract
Introduction: Data on statin safety in Asian patients are limited compared with evidence from Western populations.
Aim: This study assessed atorvastatin safety among Asian patients enrolled in 58 randomized clinical trials.
Methods: Data from 52 short-term trials (median exposure 4-72 weeks) and six long-term cardiovascular outcomes trials (median exposure 3.1-4.9 years) conducted across the atorvastatin 10-80-mg dose range were analyzed retrospectively to assess the incidence of safety endpoints.
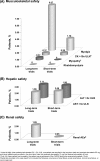
Results: A total of 77 952 patients were identified (49 974 received atorvastatin), among whom 3191 were Asian (2519 received atorvastatin). In the short-term trials, the incidence of all-causality adverse events (AEs) and serious AEs (SAEs) in Asian patients treated with atorvastatin was similar to or lower than that observed with other statins or placebo, and discontinuations due to treatment-related AEs/SAEs were infrequent (2.0% across all doses). These observations were confirmed in the long-term trials. Treatment-related SAEs were rare (n = 4) among Asian patients receiving atorvastatin. No cases of rhabdomyolysis were observed in atorvastatin-treated Asian patients, and the incidence of myalgia was 1.8% in the short-term studies and 6.7% in the long-term trials. Elevations (>3× the upper limit of normal) in liver transaminases were observed in ~2% of Asian patients receiving atorvastatin; renal AEs occurred in <2%.
Conclusion: The incidence of AEs/SAEs with atorvastatin 10-40-mg in patients of Asian origin was low and comparable to placebo. Further evaluation of atorvastatin 80-mg is required owing to the limited number of Asian patients (n = 281; 11.2%) who received this dose.
Keywords: Adverse event; Asian; Atorvastatin; Cardiovascular disease; Data pooling; Hyperlipidemia.
© 2016 The Authors Cardiovascular Therapeutics Published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Comparative safety of atorvastatin 80 mg versus 10 mg derived from analysis of 49 completed trials in 14,236 patients.Am J Cardiol. 2006 Jan 1;97(1):61-7. doi: 10.1016/j.amjcard.2005.07.108. Epub 2005 Nov 15. Am J Cardiol. 2006. PMID: 16377285
-
Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase.Lancet. 2017 Jun 24;389(10088):2473-2481. doi: 10.1016/S0140-6736(17)31075-9. Epub 2017 May 2. Lancet. 2017. PMID: 28476288 Clinical Trial.
-
Statin and the risk of renal-related serious adverse events: Analysis from the IDEAL, TNT, CARDS, ASPEN, SPARCL, and other placebo-controlled trials.Am J Cardiol. 2014 Jun 15;113(12):2018-20. doi: 10.1016/j.amjcard.2014.03.046. Epub 2014 Apr 3. Am J Cardiol. 2014. PMID: 24793673 Review.
-
Safety of atorvastatin derived from analysis of 44 completed trials in 9,416 patients.Am J Cardiol. 2003 Sep 15;92(6):670-6. doi: 10.1016/s0002-9149(03)00820-8. Am J Cardiol. 2003. PMID: 12972104
-
Efficacy of short-term high-dose atorvastatin pretreatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a meta-analysis of nine randomized controlled trials.Clin Cardiol. 2013 Dec;36(12):E41-8. doi: 10.1002/clc.22198. Epub 2013 Aug 27. Clin Cardiol. 2013. PMID: 24038054 Free PMC article. Review.
Cited by
-
Efficiency of atorvastatin on in-hospital mortality of patients with acute aortic dissection (AAD): study protocol for a randomized, open-label, superiority clinical trial.Trials. 2021 Apr 14;22(1):281. doi: 10.1186/s13063-021-05237-1. Trials. 2021. PMID: 33853639 Free PMC article.
-
Efficacy and Safety of Switching from Low-Dose Statin to High-Intensity Statin for Primary Prevention in Type 2 Diabetes: A Randomized Controlled Trial.Diabetes Metab Syndr Obes. 2020 Feb 19;13:423-431. doi: 10.2147/DMSO.S219496. eCollection 2020. Diabetes Metab Syndr Obes. 2020. PMID: 32110075 Free PMC article.
-
Roles of Achieved Levels of Low-Density Lipoprotein Cholesterol and High-Sensitivity C-Reactive Protein on Cardiovascular Outcome in Statin Therapy.Cardiovasc Ther. 2019 Nov 21;2019:3824823. doi: 10.1155/2019/3824823. eCollection 2019. Cardiovasc Ther. 2019. PMID: 31885691 Free PMC article.
-
Expert opinion on the applicability of dyslipidemia guidelines in Asia and the Middle East.Int J Gen Med. 2018 Jul 18;11:313-322. doi: 10.2147/IJGM.S160555. eCollection 2018. Int J Gen Med. 2018. PMID: 30050317 Free PMC article.
-
A randomized, open-label, parallel, multi-center Phase IV study to compare the efficacy and safety of atorvastatin 10 and 20 mg in high-risk Asian patients with hypercholesterolemia.PLoS One. 2021 Jan 22;16(1):e0245481. doi: 10.1371/journal.pone.0245481. eCollection 2021. PLoS One. 2021. PMID: 33481866 Free PMC article. Clinical Trial.
References
-
- World Health Organization [Internet] . Cardiovascular diseases (CVDs); June 2016. http://www.who.int/mediacentre/factsheets/fs317/en/index.html. Accessed July 11, 2016.
-
- World Health Organization [Internet] . The impact of chronic disease in China; October 2005. http://www.who.int/chp/chronic_disease_report/media/china.pdf. Accessed July 11, 2016.
-
- Asia Pacific Cohort Studies Collaboration . A comparison of the associations between risk factors and cardiovascular disease in Asia and Australasia. Eur J Cardiovasc Prev Rehabil. 2005;12:484–491. - PubMed
-
- Asia Pacific Cohort Studies Collaboration . Joint effects of systolic blood pressure and serum cholesterol on cardiovascular disease in the Asia Pacific region. Circulation. 2005;112:3384–3390. - PubMed
-
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

