Distinguishing the Signals of Gingivitis and Periodontitis in Supragingival Plaque: a Cross-Sectional Cohort Study in Malawi
- PMID: 27520811
- PMCID: PMC5038043
- DOI: 10.1128/AEM.01756-16
Distinguishing the Signals of Gingivitis and Periodontitis in Supragingival Plaque: a Cross-Sectional Cohort Study in Malawi
Abstract
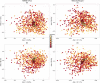
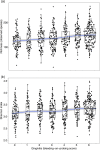
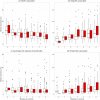
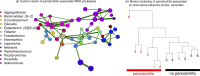
Periodontal disease ranges from gingival inflammation (gingivitis) to the inflammation and loss of tooth-supporting tissues (periodontitis). Previous research has focused mainly on subgingival plaque, but supragingival plaque composition is also known to be associated with disease. Quantitative modeling of bacterial abundances across the natural range of periodontal severities can distinguish which features of disease are associated with particular changes in composition. We assessed a cross-sectional cohort of 962 Malawian women for periodontal disease and used 16S rRNA gene amplicon sequencing (V5 to V7 region) to characterize the bacterial compositions of supragingival plaque samples. Associations between bacterial relative abundances and gingivitis/periodontitis were investigated by using negative binomial models, adjusting for epidemiological factors. We also examined bacterial cooccurrence networks to assess community structure. The main differences in supragingival plaque compositions were associated more with gingivitis than periodontitis, including higher bacterial diversity and a greater abundance of particular species. However, even after controlling for gingivitis, the presence of subgingival periodontitis was associated with an altered supragingival plaque. A small number of species were associated with periodontitis but not gingivitis, including members of Prevotella, Treponema, and Selenomonas, supporting a more complex disease model than a linear progression following gingivitis. Cooccurrence networks of periodontitis-associated taxa clustered according to periodontitis across all gingivitis severities. Species including Filifactor alocis and Fusobacterium nucleatum were central to this network, which supports their role in the coaggregation of periodontal biofilms during disease progression. Our findings confirm that periodontitis cannot be considered simply an advanced stage of gingivitis even when only considering supragingival plaque.
Importance: Periodontal disease is a major public health problem associated with oral bacteria. While earlier studies focused on a small number of periodontal pathogens, it is now accepted that the whole bacterial community may be important. However, previous high-throughput marker gene sequencing studies of supragingival plaque have largely focused on high-income populations with good oral hygiene without including a range of periodontal disease severities. Our study includes a large number of low-income participants with poor oral hygiene and a wide range of severities, and we were therefore able to quantitatively model bacterial abundances as functions of both gingivitis and periodontitis. A signal associated with periodontitis remains after controlling for gingivitis severity, which supports the concept that, even when only considering supragingival plaque, periodontitis is not simply an advanced stage of gingivitis. This suggests the future possibility of diagnosing periodontitis based on bacterial occurrences in supragingival plaque.
Copyright © 2016 Shaw et al.
Figures
Similar articles
-
Bacterial community development in experimental gingivitis.PLoS One. 2013 Aug 14;8(8):e71227. doi: 10.1371/journal.pone.0071227. eCollection 2013. PLoS One. 2013. PMID: 23967169 Free PMC article.
-
Diversity of Treponema denticola and Other Oral Treponeme Lineages in Subjects with Periodontitis and Gingivitis.Microbiol Spectr. 2021 Oct 31;9(2):e0070121. doi: 10.1128/Spectrum.00701-21. Epub 2021 Sep 29. Microbiol Spectr. 2021. PMID: 34585987 Free PMC article.
-
Salivary microbiota reflecting changes in subgingival microbiota.Microbiol Spectr. 2024 Nov 5;12(11):e0103024. doi: 10.1128/spectrum.01030-24. Epub 2024 Oct 4. Microbiol Spectr. 2024. PMID: 39365037 Free PMC article.
-
Dental calculus: recent insights into occurrence, formation, prevention, removal and oral health effects of supragingival and subgingival deposits.Eur J Oral Sci. 1997 Oct;105(5 Pt 2):508-22. doi: 10.1111/j.1600-0722.1997.tb00238.x. Eur J Oral Sci. 1997. PMID: 9395117 Review.
-
Dental bacterial plaques. Nature and role in periodontal disease.J Clin Periodontol. 1991 Jul;18(6):441-6. doi: 10.1111/j.1600-051x.1991.tb02314.x. J Clin Periodontol. 1991. PMID: 1890226 Review.
Cited by
-
Salivary proteotypes of gingivitis tolerance and resilience.J Clin Periodontol. 2020 Nov;47(11):1304-1316. doi: 10.1111/jcpe.13358. Epub 2020 Sep 16. J Clin Periodontol. 2020. PMID: 32777086 Free PMC article.
-
Tobacco smoke exacerbates Filifactor alocis pathogenicity.J Clin Periodontol. 2023 Jan;50(1):121-130. doi: 10.1111/jcpe.13729. Epub 2022 Oct 12. J Clin Periodontol. 2023. PMID: 36122937 Free PMC article.
-
Oral Dysbiosis in Severe Forms of Periodontitis Is Associated With Gut Dysbiosis and Correlated With Salivary Inflammatory Mediators: A Preliminary Study.Front Oral Health. 2021 Oct 11;2:722495. doi: 10.3389/froh.2021.722495. eCollection 2021. Front Oral Health. 2021. PMID: 35048045 Free PMC article.
-
Periodontopathogens Porphyromonas gingivalis and Fusobacterium nucleatum and Their Roles in the Progression of Respiratory Diseases.Pathogens. 2023 Aug 30;12(9):1110. doi: 10.3390/pathogens12091110. Pathogens. 2023. PMID: 37764918 Free PMC article. Review.
-
Comparison of three qPCR-based commercial tests for detection of periodontal pathogens.Sci Rep. 2021 Mar 17;11(1):6141. doi: 10.1038/s41598-021-85305-3. Sci Rep. 2021. PMID: 33731742 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

