Lipid synthesis and membrane contact sites: a crossroads for cellular physiology
- PMID: 27521373
- PMCID: PMC5036376
- DOI: 10.1194/jlr.R070920
Lipid synthesis and membrane contact sites: a crossroads for cellular physiology
Abstract
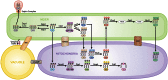
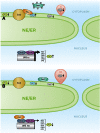
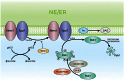
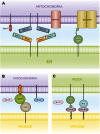
Membrane contact sites (MCSs) are regions of close apposition between different organelles that contribute to the functional integration of compartmentalized cellular processes. In recent years, we have gained insight into the molecular architecture of several contact sites, as well as into the regulatory mechanisms that underlie their roles in cell physiology. We provide an overview of two selected topics where lipid metabolism intersects with MCSs and organelle dynamics. First, the role of phosphatidic acid phosphatase, Pah1, the yeast homolog of metazoan lipin, toward the synthesis of triacylglycerol is outlined in connection with the seipin complex, Fld1/Ldb16, and lipid droplet formation. Second, we recapitulate the different contact sites connecting mitochondria and the endomembrane system and emphasize their contribution to phospholipid synthesis and their coordinated regulation. A comprehensive view is emerging where the multiplicity of contact sites connecting different cellular compartments together with lipid transfer proteins functioning at more than one MCS allow for functional redundancy and cross-regulation.
Keywords: endoplasmic reticulum; mitochondria; organelle; phosphatidic acid; triacylglycerol; vacuole.
Copyright © 2016 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Athenstaedt K., and Daum G.. 1999. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem. 266: 1–16. - PubMed
-
- Henry S. A., and Patton-Vogt J. L.. 1998. Genetic regulation of phospholipid metabolism: yeast as a model eukaryote. Prog. Nucleic Acid Res. Mol. Biol. 61: 133–179. - PubMed
-
- Travers K. J., Patil C. K., Wodicka L., Lockhart D. J., Weissman J. S., and Walter P.. 2000. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 101: 249–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

