The effect of a brief action planning intervention on adherence to double-blind study medication, compared to a standard trial protocol, in the Atorvastatin in Factorial with Omega EE90 Risk Reduction in Diabetes (AFORRD) clinical trial: A cluster randomised sub-study
- PMID: 27522034
- PMCID: PMC6078174
- DOI: 10.1016/j.diabres.2016.07.004
The effect of a brief action planning intervention on adherence to double-blind study medication, compared to a standard trial protocol, in the Atorvastatin in Factorial with Omega EE90 Risk Reduction in Diabetes (AFORRD) clinical trial: A cluster randomised sub-study
Abstract
Aims: Clinical trial patients are highly motivated but may encounter difficulty in taking study medication regularly when treatment burden is substantial. We assessed a brief behavioural intervention, given in addition to a standard trial protocol.
Methods: We performed a two-arm adherence sub-study within a twelve-month randomised controlled drug trial evaluating the impact of statin and/or omega-3 EE90 treatment in 800 patients with type 2 diabetes. Fifty-nine United Kingdom general practices were cluster-randomised to action-planning or control groups. The former delivered an initial written exercise prompting participants to formulate action-plans to take study medication regularly, with brief nurse encouragement to use action-plans at later visits, whilst the latter followed the standard trial protocol. The primary outcome was proportion of days on which study medication were taken as intended measured by electronic medication containers.
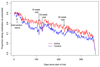
Results: Adjusted mean (95% CI) proportion of days with medication taken as intended was 79.3% (76.3-82.3%)for the 30 action-planning practices (321 participants), compared with 78.5% (75.8-81.1%) for 27 control group practices (426 participants, with a mean intervention effect of 0.9%, 95% CI -3.1% to +4.9%, p=0.67). Adjusted odds ratios for ⩾80% trial medication adherence for action-planning compared with control practices were 1.29 (0.90-1.84) and 1.38 (0.96-1.99) respectively.
Conclusions: Low-intensity action-planning interventions used alone are unlikely to have a clinically important impact on medication adherence, particularly in a clinical trial setting. These findings, do not exclude their contribution, as part of a multifactorial intervention, to improving treatment adherence. ISRCTN number 76737502.
Keywords: Action planning; Adherence; Adults; Omega-3 EE90; Statin; Type 2 diabetes.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
RRH declares research funding from Bayer, Astra Zeneca, Merck and honoraria from Amgen, Bayer, Elcelyx, Jannsen, Intarcia, Merck, Novartis, and Novo Nordisk WH has done consultancy work for AbbVie Ltd. All other authors confirm that they have no dualities of interest.
Figures
References
-
- International Diabetes Federation. IDF Diabetes Atlas. 7th Edition. Brussels, Belgium: 2015.
-
- Yach D, Stuckler D, Brownell K. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–6. - PubMed
-
- Colhoun H, Betteridge D, Durrington P, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. - PubMed
-
- Sheeran P, Orbell S. Implementation intentions and repeated behaviour: augmenting the predictive validity of the theory of planned behaviour. Eur J Soc Psychol. 1999;29:349–69.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

