POSSIBLE NATURE OF THE RADIATION-INDUCED SIGNAL IN NAILS: HIGH-FIELD EPR, CONFIRMING CHEMICAL SYNTHESIS, AND QUANTUM CHEMICAL CALCULATIONS
- PMID: 27522053
- PMCID: PMC5225972
- DOI: 10.1093/rpd/ncw216
POSSIBLE NATURE OF THE RADIATION-INDUCED SIGNAL IN NAILS: HIGH-FIELD EPR, CONFIRMING CHEMICAL SYNTHESIS, AND QUANTUM CHEMICAL CALCULATIONS
Abstract
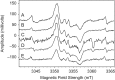
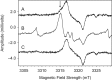
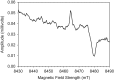
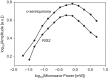
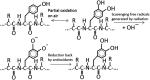
Exposure of finger- and toe-nails to ionizing radiation generates an Electron Paramagnetic Resonance (EPR) signal whose intensity is dose dependent and stable at room temperature for several days. The dependency of the radiation-induced signal (RIS) on the received dose may be used as the basis for retrospective dosimetry of an individual's fortuitous exposure to ionizing radiation. Two radiation-induced signals, a quasi-stable (RIS2) and stable signal (RIS5), have been identified in nails irradiated up to a dose of 50 Gy. Using X-band EPR, both RIS signals exhibit a singlet line shape with a line width around 1.0 mT and an apparent g-value of 2.0044. In this work, we seek information on the exact chemical nature of the radiation-induced free radicals underlying the signal. This knowledge may provide insights into the reason for the discrepancy in the stabilities of the two RIS signals and help develop strategies for stabilizing the radicals in nails or devising methods for restoring the radicals after decay. In this work an analysis of high field (94 GHz and 240 GHz) EPR spectra of the RIS using quantum chemical calculations, the oxidation-reduction properties and the pH dependence of the signal intensities are used to show that spectroscopic and chemical properties of the RIS are consistent with a semiquinone-type radical underlying the RIS. It has been suggested that semiquinone radicals formed on trace amounts of melanin in nails are the basis for the RIS signals. However, based on the quantum chemical calculations and chemical properties of the RIS, it is likely that the radicals underlying this signal are generated from the radiolysis of L-3,4-dihydroxyphenylalanine (DOPA) amino acids in the keratin proteins. These DOPA amino acids are likely formed from the exogenous oxidation of tyrosine in keratin by the oxygen from the air prior to irradiation. We show that these DOPA amino acids can work as radical traps, capturing the highly reactive and unstable sulfur-based radicals and/or alkyl radicals generated during the radiation event and are converted to the more stable o-semiquinone anion-radicals. From this understanding of the oxidation-reduction properties of the RIS, it may be possible to regenerate the unstable RIS2 following its decay through treatment of nail clippings. However, the treatment used to recover the RIS2 also has the ability to recover an interfering, mechanically-induced signal (MIS) formed when the nail is clipped. Therefore, to use the recovered (regenerated) RIS2 to increase the detection limits and precision of the RIS measurements and, therefore, the dose estimates calculated from the RIS signal amplitudes, will require the application of methods to differentiate the RIS2 from the recovered MIS signal.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
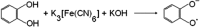
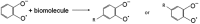
Similar articles
-
Development and validation of an ex vivo electron paramagnetic resonance fingernail biodosimetric method.Radiat Prot Dosimetry. 2014 Jun;159(1-4):172-81. doi: 10.1093/rpd/ncu129. Epub 2014 May 6. Radiat Prot Dosimetry. 2014. PMID: 24803513 Free PMC article.
-
Ex vivo analysis of irradiated fingernails: chemical yields and properties of radiation-induced and mechanically-induced radicals.Health Phys. 2010 Feb;98(2):301-8. doi: 10.1097/HP.0b013e3181b0c045. Health Phys. 2010. PMID: 20065698 Free PMC article.
-
State of the art in nail dosimetry: free radicals identification and reaction mechanisms.Radiat Environ Biophys. 2014 May;53(2):291-303. doi: 10.1007/s00411-014-0512-2. Epub 2014 Jan 28. Radiat Environ Biophys. 2014. PMID: 24469226 Free PMC article.
-
Radiation-induced signals analysed by EPR spectrometry applied to fortuitous dosimetry.Ann Ist Super Sanita. 2009;45(3):287-96. Ann Ist Super Sanita. 2009. PMID: 19861734 Review.
-
Retrospective assessment of radiation exposure using biological dosimetry: chromosome painting, electron paramagnetic resonance and the glycophorin a mutation assay.Radiat Res. 2006 Jul;166(1 Pt 2):287-302. doi: 10.1667/RR3273.1. Radiat Res. 2006. PMID: 16808614 Review.
Cited by
-
Using Stable Free Radicals to Obtain Unique and Clinically Useful Data In Vivo in Human Subjects.Radiat Prot Dosimetry. 2016 Dec;172(1-3):3-15. doi: 10.1093/rpd/ncw323. Epub 2016 Nov 24. Radiat Prot Dosimetry. 2016. PMID: 27886997 Free PMC article. Review.
-
Developments in Biodosimetry Methods for Triage With a Focus on X-band Electron Paramagnetic Resonance In Vivo Fingernail Dosimetry.Health Phys. 2018 Jul;115(1):140-150. doi: 10.1097/HP.0000000000000874. Health Phys. 2018. PMID: 29787440 Free PMC article.
-
Stability of X-band EPR signals from fingernails under vacuum storage.Radiat Phys Chem Oxf Engl 1993. 2017 Dec;141:78-87. doi: 10.1016/j.radphyschem.2017.06.009. Epub 2017 Jun 12. Radiat Phys Chem Oxf Engl 1993. 2017. PMID: 28781435 Free PMC article.
-
Behavior of the electron spin resonance signals in X-ray irradiated human fingernails for the establishment of a dose reconstruction procedure.J Radiat Res. 2021 Sep 13;62(5):812-824. doi: 10.1093/jrr/rrab027. J Radiat Res. 2021. PMID: 34095957 Free PMC article.
References
-
- Trompier F., Queinnec F., Bey E., De Revel T., Lataillade J. J., Clairand I., Benderitter M. and Bottollier-Depois J.-F.. EPR retrospective dosimetry with fingernails: report on first application cases. Health Phys. 106(6), 798–805 (2014). - PubMed
-
- Brady J. M., Aarestad N. O. and Swartz H. M.. In vivo dosimetry by electron spin resonance spectroscopy. Health Phys. 15, 43–47 (1968). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

