Sirtuin 3 Deregulation Promotes Pulmonary Fibrosis
- PMID: 27522058
- PMCID: PMC5964739
- DOI: 10.1093/gerona/glw151
Sirtuin 3 Deregulation Promotes Pulmonary Fibrosis
Abstract
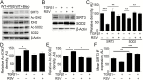
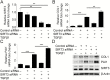
Oxidative stress leads to alveolar epithelial cell injury and fibroblast-myofibroblast differentiation (FMD), key events in the pathobiology of pulmonary fibrosis (PF). Sirtuin 3 (SIRT3) is a mitochondrial protein deacetylase regulator of antioxidant response and mitochondrial homeostasis. Here, we demonstrate reduced SIRT3 expression in the lungs of old mice compared to young mice, as well as in two murine models of PF. The analysis of the pattern of SIRT3 expression in the lungs of patients with PF revealed low SIRT3 staining within the fibrotic regions. We also demonstrated, using murine models of PF and human lung fibroblasts, that reduced SIRT3 expression in response to transforming growth factor beta 1 (TGFβ1) promotes acetylation (inactivation) of major oxidative stress response regulators, such as SOD2 and isocitrate dehydrogenase 2. Reduction of SIRT3 in human lung fibroblasts promoted FMD. By contrast, overexpression of SIRT3 attenuated TGFβ1-mediated FMD and significantly reduced the levels of SMAD family member 3 (SMAD3). Resveratrol induced SIRT3 expression and ameliorated acetylation changes induced by TGFβ1. We demonstrated that SIRT3-deficient mice are more susceptible to PF compared to control mice, and concomitantly exhibit enhanced SMAD3 expression. Collectively, these data define a SIRT3/TGFβ1 interaction during aging that may play a significant role in the pathobiology of PF.
Keywords: Age-related pathology; Lungs/pulmonary; Mitochondria; Reactive oxygen species; SIRT3.
© The Author 2016. Published by Oxford University Press on behalf of The Gerontological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




Similar articles
-
Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFβ1.Aging Cell. 2015 Oct;14(5):774-83. doi: 10.1111/acel.12357. Epub 2015 Jun 9. Aging Cell. 2015. PMID: 26059457 Free PMC article.
-
SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage.Am J Physiol Lung Cell Mol Physiol. 2017 Jan 1;312(1):L68-L78. doi: 10.1152/ajplung.00188.2016. Epub 2016 Nov 4. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 27815257 Free PMC article.
-
SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis.FASEB J. 2017 Jun;31(6):2520-2532. doi: 10.1096/fj.201601077R. Epub 2017 Mar 3. FASEB J. 2017. PMID: 28258190 Free PMC article.
-
The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis.Int J Mol Sci. 2015 Sep 7;16(9):21486-519. doi: 10.3390/ijms160921486. Int J Mol Sci. 2015. PMID: 26370974 Free PMC article. Review.
-
Manganese Superoxide Dismutase Acetylation and Dysregulation, Due to Loss of SIRT3 Activity, Promote a Luminal B-Like Breast Carcinogenic-Permissive Phenotype.Antioxid Redox Signal. 2016 Aug 20;25(6):326-36. doi: 10.1089/ars.2016.6641. Epub 2016 Apr 15. Antioxid Redox Signal. 2016. PMID: 26935174 Free PMC article. Review.
Cited by
-
SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis.Oncotarget. 2016 Oct 25;7(43):69321-69336. doi: 10.18632/oncotarget.12504. Oncotarget. 2016. PMID: 27732568 Free PMC article.
-
Role of Carbon Monoxide in Oxidative Stress-Induced Senescence in Human Bronchial Epithelium.Oxid Med Cell Longev. 2022 Sep 24;2022:5199572. doi: 10.1155/2022/5199572. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36193088 Free PMC article.
-
SIRT3 deficiency leads to induction of abnormal glycolysis in diabetic kidney with fibrosis.Cell Death Dis. 2018 Sep 24;9(10):997. doi: 10.1038/s41419-018-1057-0. Cell Death Dis. 2018. PMID: 30250024 Free PMC article.
-
PINK1-PARK2-mediated mitophagy in COPD and IPF pathogeneses.Inflamm Regen. 2018 Oct 24;38:18. doi: 10.1186/s41232-018-0077-6. eCollection 2018. Inflamm Regen. 2018. PMID: 30386443 Free PMC article. Review.
-
Cellular Senescence in Aging Lungs and Diseases.Cells. 2022 May 29;11(11):1781. doi: 10.3390/cells11111781. Cells. 2022. PMID: 35681476 Free PMC article. Review.
References
-
- Kawakami T, Ihn H, Xu W, Smith E, LeRoy C, Trojanowska M. Increased expression of TGF-beta receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-beta signaling to scleroderma phenotype. J Invest Dermatol. 1998;110:47–51. doi:10.1046/j.1523-1747.1998.00073.x - PubMed
-
- Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18–64 years old. Eur Respir J. 2016;48:179–186. doi:10.1183/13993003.01653-2015 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

