Vascular, glial, and lymphatic immune gateways of the central nervous system
- PMID: 27522506
- PMCID: PMC4992028
- DOI: 10.1007/s00401-016-1606-5
Vascular, glial, and lymphatic immune gateways of the central nervous system
Abstract
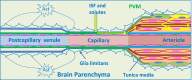
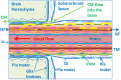
Immune privilege of the central nervous system (CNS) has been ascribed to the presence of a blood-brain barrier and the lack of lymphatic vessels within the CNS parenchyma. However, immune reactions occur within the CNS and it is clear that the CNS has a unique relationship with the immune system. Recent developments in high-resolution imaging techniques have prompted a reassessment of the relationships between the CNS and the immune system. This review will take these developments into account in describing our present understanding of the anatomical connections of the CNS fluid drainage pathways towards regional lymph nodes and our current concept of immune cell trafficking into the CNS during immunosurveillance and neuroinflammation. Cerebrospinal fluid (CSF) and interstitial fluid are the two major components that drain from the CNS to regional lymph nodes. CSF drains via lymphatic vessels and appears to carry antigen-presenting cells. Interstitial fluid from the CNS parenchyma, on the other hand, drains to lymph nodes via narrow and restricted basement membrane pathways within the walls of cerebral capillaries and arteries that do not allow traffic of antigen-presenting cells. Lymphocytes targeting the CNS enter by a two-step process entailing receptor-mediated crossing of vascular endothelium and enzyme-mediated penetration of the glia limitans that covers the CNS. The contribution of the pathways into and out of the CNS as initiators or contributors to neurological disorders, such as multiple sclerosis and Alzheimer's disease, will be discussed. Furthermore, we propose a clear nomenclature allowing improved precision when describing the CNS-specific communication pathways with the immune system.
Keywords: Alzheimer’s disease; Antigen-presenting cells; Blood–brain barrier; CNS; CSF; Dendritic cells; Glia limitans: multiple sclerosis; Immune privilege; Interstitial fluid; Lymphatic drainage.
Figures




References
-
- Abadier M, Haghayegh Jahromi N, Cardoso Alves L, Boscacci R, Vestweber D, Barnum S, Deutsch U, Engelhardt B, Lyck R. Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood–brain barrier. Eur J Immunol. 2015;45:1043–1058. doi: 10.1002/eji.201445125. - DOI - PubMed
-
- Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

