Rationale and Design of the ATHENA-HF Trial: Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure
- PMID: 27522631
- PMCID: PMC5010507
- DOI: 10.1016/j.jchf.2016.06.003
Rationale and Design of the ATHENA-HF Trial: Aldosterone Targeted Neurohormonal Combined With Natriuresis Therapy in Heart Failure
Abstract
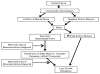
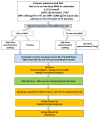
Although therapy with mineralocorticoid receptor antagonists (MRAs) is recommended for patients with chronic heart failure (HF) with reduced ejection fraction and in post-infarction HF, it has not been studied well in acute HF (AHF) despite being commonly used in this setting. At high doses, MRA therapy in AHF may relieve congestion through its natriuretic properties and mitigate the effects of adverse neurohormonal activation associated with intravenous loop diuretics. The ATHENA-HF (Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure) trial is a randomized, double-blind, placebo-controlled study of the safety and efficacy of 100 mg/day spironolactone versus placebo (or continued low-dose spironolactone use in participants who are already receiving spironolactone at baseline) in 360 patients hospitalized for AHF. Patients are randomized within 24 h of receiving the first dose of intravenous diuretics. The primary objective is to determine if high-dose spironolactone, compared with standard care, will lead to greater reductions in N-terminal pro-B-type natriuretic peptide levels from randomization to 96 h. The secondary endpoints include changes in the clinical congestion score, dyspnea relief, urine output, weight change, loop diuretic dose, and in-hospital worsening HF. Index hospital length of stay and 30-day clinical outcomes will be assessed. Safety endpoints include risk of hyperkalemia and renal function. Differences among patients with reduced versus preserved ejection fraction will be determined. (Study of High-dose Spironolactone vs. Placebo Therapy in Acute Heart Failure [ATHENA-HF]; NCT02235077).
Keywords: acute heart failure; aldosterone; heart failure; hospitalization; mineralocorticoid receptor antagonist; natriuretic peptides.
Copyright © 2016 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. - PubMed
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. - PubMed
-
- Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr, Gheorghiade M, O'Connor CM. Risk stratification after hospitalization for decompensated heart failure. Journal of cardiac failure. 2004;10:460–6. - PubMed
-
- Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. The New England journal of medicine. 2010;363:1419–28. - PubMed
-
- Chin MH, Goldman L. Correlates of early hospital readmission or death in patients with congestive heart failure. The American journal of cardiology. 1997;79:1640–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 HL084890/HL/NHLBI NIH HHS/United States
- U01 HL084891/HL/NHLBI NIH HHS/United States
- U01 HL084931/HL/NHLBI NIH HHS/United States
- U01 HL084875/HL/NHLBI NIH HHS/United States
- U10 HL110312/HL/NHLBI NIH HHS/United States
- U01 HL084907/HL/NHLBI NIH HHS/United States
- U01 HL084899/HL/NHLBI NIH HHS/United States
- U10 HL110302/HL/NHLBI NIH HHS/United States
- U01 HL084861/HL/NHLBI NIH HHS/United States
- U10 HL084904/HL/NHLBI NIH HHS/United States
- U01 HL084877/HL/NHLBI NIH HHS/United States
- U01 HL084889/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

