Hunger-Dependent Enhancement of Food Cue Responses in Mouse Postrhinal Cortex and Lateral Amygdala
- PMID: 27523426
- PMCID: PMC5017916
- DOI: 10.1016/j.neuron.2016.07.032
Hunger-Dependent Enhancement of Food Cue Responses in Mouse Postrhinal Cortex and Lateral Amygdala
Abstract
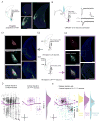
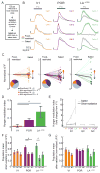
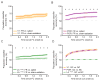
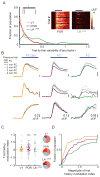
The needs of the body can direct behavioral and neural processing toward motivationally relevant sensory cues. For example, human imaging studies have consistently found specific cortical areas with biased responses to food-associated visual cues in hungry subjects, but not in sated subjects. To obtain a cellular-level understanding of these hunger-dependent cortical response biases, we performed chronic two-photon calcium imaging in postrhinal association cortex (POR) and primary visual cortex (V1) of behaving mice. As in humans, neurons in mouse POR, but not V1, exhibited biases toward food-associated cues that were abolished by satiety. This emergent bias was mirrored by the innervation pattern of amygdalo-cortical feedback axons. Strikingly, these axons exhibited even stronger food cue biases and sensitivity to hunger state and trial history. These findings highlight a direct pathway by which the lateral amygdala may contribute to state-dependent cortical processing of motivationally relevant sensory cues.
Published by Elsevier Inc.
Figures



References
-
- Badre D, Frank MJ, Moore CI. Interactionist Neuroscience. Neuron. 2015;88:855–860. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

