Intra-cluster and inter-period correlation coefficients for cross-sectional cluster randomised controlled trials for type-2 diabetes in UK primary care
- PMID: 27524396
- PMCID: PMC4983799
- DOI: 10.1186/s13063-016-1532-9
Intra-cluster and inter-period correlation coefficients for cross-sectional cluster randomised controlled trials for type-2 diabetes in UK primary care
Abstract
Background: Clustered randomised controlled trials (CRCTs) are increasingly common in primary care. Outcomes within the same cluster tend to be correlated with one another. In sample size calculations, estimates of the intra-cluster correlation coefficient (ICC) are needed to allow for this nonindependence. In studies with observations over more than one time period, estimates of the inter-period correlation (IPC) and the within-period correlation (WPC) are also needed.
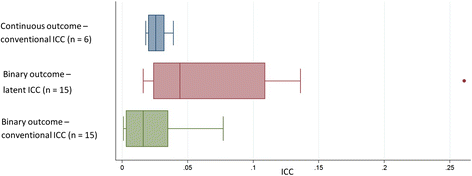
Methods: This is a retrospective cross-sectional study of all patients aged 18 or over with a diagnosis of type-2 diabetes, from The Health Improvement Network (THIN) database, between 1 October 2007 and 31 March 2010. We report estimates of the ICC, IPC, and WPC for typical outcomes using unadjusted and adjusted generalised linear mixed models with cluster and cluster by period random effects. For binary outcomes we report on the proportions scale, which is the appropriate scale for trial design. Estimated ICCs were compared to those reported from a systematic search of CRCTs undertaken in primary care in the UK in type-2 diabetes.
Results: Data from 430 general practices, with a median [IQR] number of diabetics per practice of 241 [150-351], were analysed. The ICC for HbA1c was 0.032 (95 % CI 0.026-0.038). For a two-period (each of 12 months) design, the WPC for HbA1c was 0.035 (95 % CI 0.030-0.040) and the IPC was 0.019 (95 % CI 0.014-0.026). The difference between the WPC and the IPC indicates a decay of correlation over time. Following dichotomisation at 7.5 %, the ICC for HbA1c was 0.026 (95 % CI 0.022-0.030). ICCs for other clinical measurements and clinical outcomes are presented. A systematic search of ICCs used in the design of CRCTs involving type-2 diabetes with HbA1c (undichotomised) as the outcome found that published trials tended to use more conservative ICC values (median 0.047, IQR 0.047-0.050) than those reported here.
Conclusions: These estimates of ICCs, IPCs, and WPCs for a variety of outcomes commonly used in diabetes trials can be useful for the design of CRCTs. In studies with observations taken at different time-points, the correlation of observations may decay over time, as reflected in lower values for the IPC than for the ICC. The IPC and WPC estimates are the first reported for UK primary care data.
Keywords: Cluster randomised trial; Inter-period correlation coefficient; Intra-cluster correlation coefficient; Type-2 diabetes.
Figures
References
-
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. doi: 10.1016/S0140-6736(11)60679-X. - DOI - PubMed
-
- Donner A, Kong AP. Design and analysis of cluster randomization trials in health research. London: Arnold Publishers Limited; 2000.
-
- Donner A. Some aspects of the design and analysis of cluster randomization trials. J R Stat Soc: Ser C: Appl Stat. 1998;47(1):95–113. doi: 10.1111/1467-9876.00100. - DOI
-
- Campbell MJ, Walters SJ. How to design, analyse and report cluster randomised trials in medicine and health related research. West Sussex: John Wiley & Sons Ltd, Wiley; 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical