Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes
- PMID: 27524410
- PMCID: PMC5048597
- DOI: 10.1016/j.mce.2016.08.006
Bioactive forms of vitamin D selectively stimulate the skin analog of the hypothalamus-pituitary-adrenal axis in human epidermal keratinocytes
Abstract
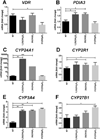
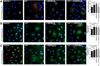
Ultraviolet radiation B stimulates both the production of vitamin D3 in the skin and the activation of the skin analog of the hypothalamic-pituitary-adrenal axis (HPA) as well as the central HPA. Since the role of vitamin D3 in the regulation of the HPA is largely unknown, we investigated the impact of 1,25(OH)2D3 and its noncalcemic analogs, 20(OH)D3 and 21(OH)pD, on the expression of the local HPA in human epidermal keratinocytes. The noncalcemic analogs showed similar efficacy to 1,25(OH)2D3 in stimulating the expression of neuropeptides, CRF, urocortins and POMC, and their receptors, CRFR1, CRFR2, MC1R, MC2R, MC3R and MC4R. Interestingly, unlike other secosteroids, the activity of 21(OH)pD did not correlate with induction of differentiation, suggesting a separate but overlapping mechanism of action. Thus, biologically active forms of vitamin D can regulate different elements of the local equivalent of the HPA with implications for the systemic HPA.
Keywords: Calcium; Corticotropin releasing factor; HPA axis; Keratinocytes differentiation; Vitamin D(3); Vitamin D(3) analogs.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures





Similar articles
-
Differentiation of Keratinocytes Modulates Skin HPA Analog.J Cell Physiol. 2017 Jan;232(1):154-66. doi: 10.1002/jcp.25400. Epub 2016 Aug 10. J Cell Physiol. 2017. PMID: 27061711
-
Effects of vitamin D metabolites on proliferation and differentiation of cultured human epidermal keratinocytes grown in serum-free or defined culture medium.Endocrinology. 1994 Nov;135(5):1793-8. doi: 10.1210/endo.135.5.7956903. Endocrinology. 1994. PMID: 7956903
-
Challenge and perspective: the relevance of ultraviolet (UV) radiation and the vitamin D endocrine system (VDES) for psoriasis and other inflammatory skin diseases.Photochem Photobiol Sci. 2017 Mar 16;16(3):433-444. doi: 10.1039/c6pp00280c. Photochem Photobiol Sci. 2017. PMID: 28054069 Review.
-
Vitamin D and epidermal differentiation: evidence for a role of endogenously produced vitamin D metabolites in keratinocyte differentiation.Skin Pharmacol. 1988;1(3):149-60. doi: 10.1159/000210769. Skin Pharmacol. 1988. PMID: 3078531 Review.
-
Vitamin D derivatives enhance cytotoxic effects of H2O2 or cisplatin on human keratinocytes.Steroids. 2016 Jun;110:49-61. doi: 10.1016/j.steroids.2016.04.002. Epub 2016 Apr 13. Steroids. 2016. PMID: 27083311 Free PMC article.
Cited by
-
Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system.Proc Natl Acad Sci U S A. 2024 Apr 2;121(14):e2308374121. doi: 10.1073/pnas.2308374121. Epub 2024 Mar 15. Proc Natl Acad Sci U S A. 2024. PMID: 38489380 Free PMC article.
-
Illuminating the Connection: Cutaneous Vitamin D3 Synthesis and Its Role in Skin Cancer Prevention.Nutrients. 2025 Jan 22;17(3):386. doi: 10.3390/nu17030386. Nutrients. 2025. PMID: 39940244 Free PMC article. Review.
-
Beneficial Regulation of Cellular Oxidative Stress Effects, and Expression of Inflammatory, Angiogenic, and the Extracellular Matrix Remodeling Proteins by 1α,25-Dihydroxyvitamin D3 in a Melanoma Cell Line.Molecules. 2020 Mar 5;25(5):1164. doi: 10.3390/molecules25051164. Molecules. 2020. PMID: 32150881 Free PMC article.
-
Biological Effects of CYP11A1-Derived Vitamin D and Lumisterol Metabolites in the Skin.J Invest Dermatol. 2024 Oct;144(10):2145-2161. doi: 10.1016/j.jid.2024.04.022. Epub 2024 Jul 12. J Invest Dermatol. 2024. PMID: 39001720 Review.
-
Serum Vitamin D Levels in Children and Adolescents with Vasovagal Syncope, Syncope Due to Orthostatic Hypotension, and Cardiac Syncope.Turk Arch Pediatr. 2023 Jan;58(1):42-48. doi: 10.5152/TurkArchPediatr.2022.22141. Turk Arch Pediatr. 2023. PMID: 36598210 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

