A Link between Integral Membrane Protein Expression and Simulated Integration Efficiency
- PMID: 27524616
- PMCID: PMC5001948
- DOI: 10.1016/j.celrep.2016.07.042
A Link between Integral Membrane Protein Expression and Simulated Integration Efficiency
Abstract
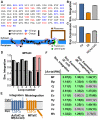
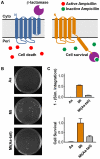
Integral membrane proteins (IMPs) control the flow of information and nutrients across cell membranes, yet IMP mechanistic studies are hindered by difficulties in expression. We investigate this issue by addressing the connection between IMP sequence and observed expression levels. For homologs of the IMP TatC, observed expression levels vary widely and are affected by small changes in protein sequence. The effect of sequence changes on experimentally observed expression levels strongly correlates with the simulated integration efficiency obtained from coarse-grained modeling, which is directly confirmed using an in vivo assay. Furthermore, mutations that improve the simulated integration efficiency likewise increase the experimentally observed expression levels. Demonstration of these trends in both Escherichia coli and Mycobacterium smegmatis suggests that the results are general to other expression systems. This work suggests that IMP integration is a determinant for successful expression, raising the possibility of controlling IMP expression via rational design.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Genomic organization and in vivo characterization of proteolytic activity of FtsH of Mycobacterium smegmatis SN2.Microbiology (Reading). 2004 Aug;150(Pt 8):2629-2639. doi: 10.1099/mic.0.27090-0. Microbiology (Reading). 2004. PMID: 15289559
-
The crystal structure of the mycobacterial trehalose monomycolate transport factor A, TtfA, reveals an atypical fold.Proteins. 2020 Jun;88(6):809-815. doi: 10.1002/prot.25863. Epub 2019 Dec 19. Proteins. 2020. PMID: 31833106
-
Improving membrane protein expression by optimizing integration efficiency.J Biol Chem. 2017 Nov 24;292(47):19537-19545. doi: 10.1074/jbc.M117.813469. Epub 2017 Sep 16. J Biol Chem. 2017. PMID: 28918393 Free PMC article.
-
Assembly of membrane-bound respiratory complexes by the Tat protein-transport system.Arch Microbiol. 2002 Aug;178(2):77-84. doi: 10.1007/s00203-002-0434-2. Epub 2002 May 22. Arch Microbiol. 2002. PMID: 12115052 Review.
-
Biogenesis of inner membrane proteins in Escherichia coli.Annu Rev Microbiol. 2005;59:329-55. doi: 10.1146/annurev.micro.59.030804.121246. Annu Rev Microbiol. 2005. PMID: 16153172 Review.
Cited by
-
Contribution of Cotranslational Folding Defects to Membrane Protein Homeostasis.J Am Chem Soc. 2019 Jan 9;141(1):204-215. doi: 10.1021/jacs.8b08243. Epub 2018 Dec 26. J Am Chem Soc. 2019. PMID: 30537820 Free PMC article.
-
Dynamics of Co-translational Membrane Protein Integration and Translocation via the Sec Translocon.J Am Chem Soc. 2020 Mar 25;142(12):5449-5460. doi: 10.1021/jacs.9b07820. Epub 2020 Mar 13. J Am Chem Soc. 2020. PMID: 32130863 Free PMC article. Review.
-
Molecular basis for the evolved instability of a human G-protein coupled receptor.Cell Rep. 2021 Nov 23;37(8):110046. doi: 10.1016/j.celrep.2021.110046. Cell Rep. 2021. PMID: 34818554 Free PMC article.
-
Emerging Diversity in Lipid-Protein Interactions.Chem Rev. 2019 May 8;119(9):5775-5848. doi: 10.1021/acs.chemrev.8b00451. Epub 2019 Feb 13. Chem Rev. 2019. PMID: 30758191 Free PMC article. Review.
-
A statistical model for improved membrane protein expression using sequence-derived features.J Biol Chem. 2018 Mar 30;293(13):4913-4927. doi: 10.1074/jbc.RA117.001052. Epub 2018 Jan 29. J Biol Chem. 2018. PMID: 29378850 Free PMC article.
References
-
- BOGSCH EG, SARGENT F, STANLEY NR, BERKS BC, ROBINSON C, PALMER T. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J Biol Chem. 1998;273:18003–6. - PubMed
-
- DALEY DO, RAPP M, GRANSETH E, MELÉN K, DREW D, HEIJNE GV. Global Topology Analysis of the Escherichia coli Inner Membrane Proteome. Science. 2005;308:1321–1323. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

