Synthesis, characterization and monoamine transporter activity of the new psychoactive substance mexedrone and its N-methoxy positional isomer, N-methoxymephedrone
- PMID: 27524685
- PMCID: PMC5336524
- DOI: 10.1002/dta.2053
Synthesis, characterization and monoamine transporter activity of the new psychoactive substance mexedrone and its N-methoxy positional isomer, N-methoxymephedrone
Abstract
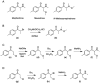
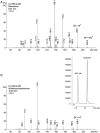
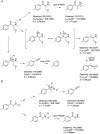
3-Methoxy-2-(methylamino)-1-(4-methylphenyl)propan-1-one (mexedrone) appeared in 2015 and was advertised by UK Internet retailers as a non-controlled mephedrone derivative (2-(methylamino)-1-(4-methylphenyl)propan-1-one), which was of particular interest to countries who operate generic drugs legislation. This study describes the synthesis and analytical characterization of mexedrone and the differentiation from its isomer, N-methoxymephedrone, which was predicted to be a suitable candidate before the identity of mexedrone was revealed. A full analytical characterization is described using various chromatographic, spectroscopic and mass spectrometric platforms and X-ray crystal structure analysis. The analytical data obtained for a vendor sample were consistent with the synthesized mexedrone reference standard and analytical differentiation between the mexedrone and N-methoxymephedrone positional isomers was achieved. Furthermore, α-chloromethylmephedrone was identified as a by-product during mexedrone synthesis. All three substances were also studied for their uptake and releasing properties at dopamine transporters (DAT), norepinephrine transporters (NET) and serotonin transporters (SERT) using in vitro monoamine transporter assays in rat brain synaptosomes and compared to mephedrone. Mexedrone was a weak non-selective uptake blocker with IC50 values in the low μM range. It was also devoid of releasing activity at DAT and NET but displayed weak releasing activity at SERT (EC50 = 2.5 μM). The isomer N-methoxymephedrone was found to be a weak uptake blocker at DAT, NET and SERT, as well as a fully efficacious substrate-type releasing agent across all three transporters with EC50 values in the low micromolar range. The synthesis by-product α-chloromethylmephedrone was inactive in all assays. Copyright © 2016 John Wiley & Sons, Ltd.
Keywords: chemistry; mephedrone; mexedrone; new psychoactive substances; psychostimulants.
Copyright © 2016 John Wiley & Sons, Ltd.
Figures



References
-
- Council of the European Union. Council Decision of 2 December 2010 on submitting 4-methylmethcathinone (mephedrone) to control measures (2010/759/EU) Off J European Union. 2010;L322:44. Available at: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2010.32... [18 June 2016]
-
- United Nations Office on Drugs and Crime. Inclusion of mephedrone (4-methylmethcathinone) in Schedule II of the Convention on Psychotropic Substances of 1971. 2015 Available at: https://www.unodc.org/documents/commissions/CND/CND_Sessions/CND_58/2015... [20 June 2016]
-
- de Burnaga Sanchez MJS. Sur un homologue de l'éphédrine. Bull Soc Chim Fr. 1929;45:284.
-
- McElrath K, O'Neill C. Experiences with mephedrone pre and post legislative controls, perceptions of safety and sources of supply. Int J Drug Policy. 2011;22:120. - PubMed
-
- Van Hout MC, Brennan R. Plant food for thought: A quantitative study of mephedrone in Ireland. Drugs Educ Prev Pol. 2011;18:371.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

