Restricting retrotransposons: a review
- PMID: 27525044
- PMCID: PMC4982230
- DOI: 10.1186/s13100-016-0070-z
Restricting retrotransposons: a review
Abstract
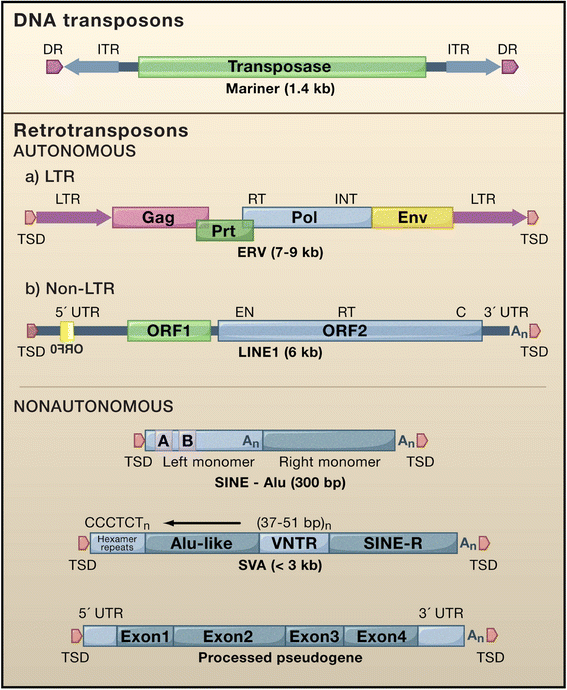
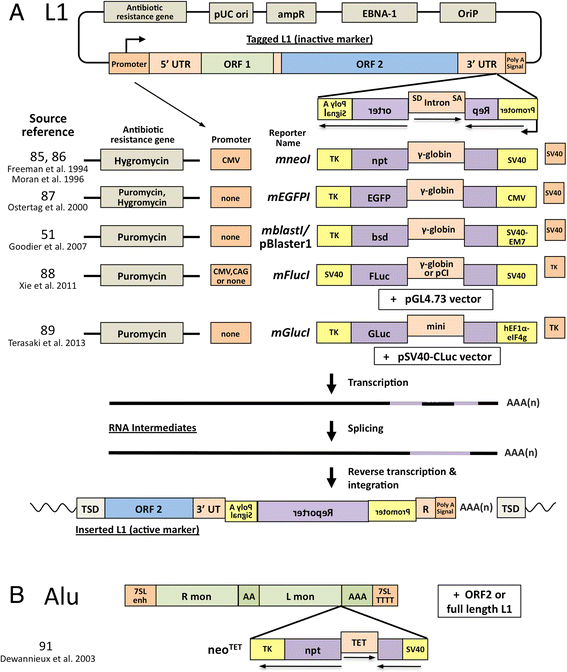
Retrotransposons have generated about 40 % of the human genome. This review examines the strategies the cell has evolved to coexist with these genomic "parasites", focussing on the non-long terminal repeat retrotransposons of humans and mice. Some of the restriction factors for retrotransposition, including the APOBECs, MOV10, RNASEL, SAMHD1, TREX1, and ZAP, also limit replication of retroviruses, including HIV, and are part of the intrinsic immune system of the cell. Many of these proteins act in the cytoplasm to degrade retroelement RNA or inhibit its translation. Some factors act in the nucleus and involve DNA repair enzymes or epigenetic processes of DNA methylation and histone modification. RISC and piRNA pathway proteins protect the germline. Retrotransposon control is relaxed in some cell types, such as neurons in the brain, stem cells, and in certain types of disease and cancer, with implications for human health and disease. This review also considers potential pitfalls in interpreting retrotransposon-related data, as well as issues to consider for future research.
Keywords: Alu; Autoimmunity; Epigenetics; LINE-1; Methylation; RNAi; Restriction; Retrovirus; SINE; SVA.
Figures

References
-
- Kazazian HH. Mobile DNA: Finding Treasure in Junk. New Jersey: FT Press, Upper Saddle River; 2011.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

