Dengue Fever: Causes, Complications, and Vaccine Strategies
- PMID: 27525287
- PMCID: PMC4971387
- DOI: 10.1155/2016/6803098
Dengue Fever: Causes, Complications, and Vaccine Strategies
Abstract
Dengue is a highly endemic infectious disease of the tropical countries and is rapidly becoming a global burden. It is caused by any of the 4 serotypes of dengue virus and is transmitted within humans through female Aedes mosquitoes. Dengue disease varies from mild fever to severe conditions of dengue hemorrhagic fever and shock syndrome. Globalization, increased air travel, and unplanned urbanization have led to increase in the rate of infection and helped dengue to expand its geographic and demographic distribution. Dengue vaccine development has been a challenging task due to the existence of four antigenically distinct dengue virus serotypes, each capable of eliciting cross-reactive and disease-enhancing antibody response against the remaining three serotypes. Recently, Sanofi Pasteur's chimeric live-attenuated dengue vaccine candidate has been approved in Mexico, Brazil, and Philippines for usage in adults between 9 and 45 years of age. The impact of its limited application to the public health system needs to be evaluated. Simultaneously, the restricted application of this vaccine candidate warrants continued efforts in developing a dengue vaccine candidate which is additionally efficacious for infants and naïve individuals. In this context, alternative strategies of developing a designed vaccine candidate which does not allow production of enhancing antibodies should be explored, as it may expand the umbrella of efficacy to include infants and naïve individuals.
Figures

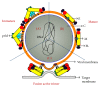



References
-
- Sanofi Pasteur Media Release. http://www.sanofipasteur.com/en/articles/First-Vaccinations-against-Deng....
-
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland: WHO; 2009. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

