Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer
- PMID: 27525306
- PMCID: PMC4972091
- DOI: 10.1038/mto.2016.18
Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer
Abstract
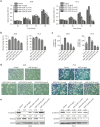
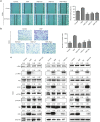
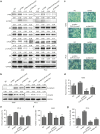
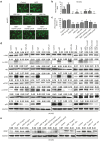
The epithelial-mesenchymal transition (EMT) and angiogenesis have emerged as two pivotal events in cancer progression. Curcumin has been extensively studied in preclinical models and clinical trials of cancer prevention due to its favorable toxicity profile. However, the possible involvement of curcumin in the EMT and angiogenesis in lung cancer remains unclear. This study found that curcumin inhibited hepatocyte growth factor (HGF)-induced migration and EMT-related morphological changes in A549 and PC-9 cells. Moreover, pretreatment with curcumin blocked HGF-induced c-Met phosphorylation and downstream activation of Akt, mTOR, and S6. These effects mimicked that of c-Met inhibitor SU11274 or PI3 kinase inhibitor LY294002 or mTOR inhibitor rapamycin treatment. c-Met gene overexpression analysis further demonstrated that curcumin suppressed lung cancer cell EMT by inhibiting c-Met/Akt/mTOR signaling pathways. In human umbilical vein endothelial cells (HUVECs), we found that curcumin also significantly inhibited PI3K/Akt/mTOR signaling and induced apoptosis and reduced migration and tube formation of HGF-treated HUVEC. Finally, in the experimental mouse model, we showed that curcumin inhibited HGF-stimulated tumor growth and induced an increase in E-cadherin expression and a decrease in vimentin, CD34, and vascular endothelial growth factor (VEGF) expression. Collectively, these findings indicated that curcumin could inhibit HGF-promoted EMT and angiogenesis by targeting c-Met and blocking PI3K/Akt/mTOR pathways.
Figures

Similar articles
-
MiR-206 inhibits HGF-induced epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via c-Met /PI3k/Akt/mTOR pathway.Oncotarget. 2016 Apr 5;7(14):18247-61. doi: 10.18632/oncotarget.7570. Oncotarget. 2016. PMID: 26919096 Free PMC article.
-
Curcumin Inhibits HGF-Induced EMT by Regulating c-MET-Dependent PI3K/Akt/mTOR Signaling Pathways in Meningioma.Evid Based Complement Alternat Med. 2021 Aug 6;2021:5574555. doi: 10.1155/2021/5574555. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34408780 Free PMC article.
-
Shikonin Inhibited Migration and Invasion of Human Lung Cancer Cells via Suppression of c-Met-Mediated Epithelial-to-Mesenchymal Transition.J Cell Biochem. 2017 Dec;118(12):4639-4651. doi: 10.1002/jcb.26128. Epub 2017 Jun 19. J Cell Biochem. 2017. PMID: 28485480
-
Role of HGF/MET axis in resistance of lung cancer to contemporary management.Transl Lung Cancer Res. 2012 Sep;1(3):179-93. doi: 10.3978/j.issn.2218-6751.2012.09.04. Transl Lung Cancer Res. 2012. PMID: 25806180 Free PMC article. Review.
-
Hepatocyte Growth Factor, a Key Tumor-Promoting Factor in the Tumor Microenvironment.Cancers (Basel). 2017 Apr 17;9(4):35. doi: 10.3390/cancers9040035. Cancers (Basel). 2017. PMID: 28420162 Free PMC article. Review.
Cited by
-
Unveiling the power of flavonoids: A dynamic exploration of their impact on cancer through matrix metalloproteinases regulation.Biomedicine (Taipei). 2024 Jun 1;14(2):12-28. doi: 10.37796/2211-8039.1447. eCollection 2024. Biomedicine (Taipei). 2024. PMID: 38939095 Free PMC article. Review.
-
Study Deciphering the Crucial Involvement of Notch Signaling Pathway in Human Cancers.Endocr Metab Immune Disord Drug Targets. 2024;24(11):1241-1253. doi: 10.2174/0118715303261691231107113548. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 37997805 Review.
-
Anti-Cancer and Ototoxicity Characteristics of the Curcuminoids, CLEFMA and EF24, in Combination with Cisplatin.Molecules. 2019 Oct 29;24(21):3889. doi: 10.3390/molecules24213889. Molecules. 2019. PMID: 31671767 Free PMC article.
-
The roles of curcumin in regulating the tumor immunosuppressive microenvironment.Oncol Lett. 2020 Apr;19(4):3059-3070. doi: 10.3892/ol.2020.11437. Epub 2020 Mar 3. Oncol Lett. 2020. PMID: 32256807 Free PMC article. Review.
-
Role of matrix metalloproteinase-9 in transforming growth factor-β1-induced epithelial-mesenchymal transition in esophageal squamous cell carcinoma.Onco Targets Ther. 2017 Jun 2;10:2837-2847. doi: 10.2147/OTT.S134813. eCollection 2017. Onco Targets Ther. 2017. PMID: 28652766 Free PMC article.
References
-
- Meng, F and Wu, G (2012). The rejuvenated scenario of epithelial-mesenchymal transition (EMT) and cancer metastasis. Cancer Metastasis Rev 31: 455–467. - PubMed
-
- Gentile, A, Trusolino, L and Comoglio, PM (2008). The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev 27: 85–94. - PubMed
-
- Feng, Y, Minca, EC, Lanigan, C, Liu, A, Zhang, W, Yin, L et al. (2014). High MET receptor expression but not gene amplification in ALK 2p23 rearrangement positive non-small-cell lung cancer. J Thorac Oncol 9: 646–653. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

