Lymphatic vessels regulate immune microenvironments in human and murine melanoma
- PMID: 27525437
- PMCID: PMC5004967
- DOI: 10.1172/JCI79434
Lymphatic vessels regulate immune microenvironments in human and murine melanoma
Abstract
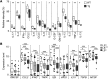
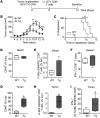
Lymphatic remodeling in tumor microenvironments correlates with progression and metastasis, and local lymphatic vessels play complex and poorly understood roles in tumor immunity. Tumor lymphangiogenesis is associated with increased immune suppression, yet lymphatic vessels are required for fluid drainage and immune cell trafficking to lymph nodes, where adaptive immune responses are mounted. Here, we examined the contribution of lymphatic drainage to tumor inflammation and immunity using a mouse model that lacks dermal lymphatic vessels (K14-VEGFR3-Ig mice). Melanomas implanted in these mice grew robustly, but exhibited drastically reduced cytokine expression and leukocyte infiltration compared with those implanted in control animals. In the absence of local immune suppression, transferred cytotoxic T cells more effectively controlled tumors in K14-VEGFR3-Ig mice than in control mice. Furthermore, gene expression analysis of human melanoma samples revealed that patient immune parameters are markedly stratified by levels of lymphatic markers. This work suggests that the establishment of tumor-associated inflammation and immunity critically depends on lymphatic vessel remodeling and drainage. Moreover, these results have implications for immunotherapies, the efficacies of which are regulated by the tumor immune microenvironment.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

