Frontal Traumatic Brain Injury in Rats Causes Long-Lasting Impairments in Impulse Control That Are Differentially Sensitive to Pharmacotherapeutics and Associated with Chronic Neuroinflammation
- PMID: 27525447
- PMCID: PMC9487719
- DOI: 10.1021/acschemneuro.6b00166
Frontal Traumatic Brain Injury in Rats Causes Long-Lasting Impairments in Impulse Control That Are Differentially Sensitive to Pharmacotherapeutics and Associated with Chronic Neuroinflammation
Abstract
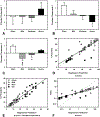
Traumatic brain injury (TBI) affects millions yearly, and is increasingly associated with chronic neuropsychiatric symptoms. We assessed the long-term effects of different bilateral frontal controlled cortical impact injury severities (mild, moderate, and severe) on the five-choice serial reaction time task, a paradigm with relatively independent measurements of attention, motor impulsivity, and motivation. Moderately- and severely injured animals exhibited impairments across all cognitive domains that were still evident 14 weeks postinjury, while mild-injured animals only demonstrated persistent deficits in impulse control. However, recovery of function varied considerably between subjects such that some showed no impairment ("TBI-resilient"), some demonstrated initial deficits that recovered ("TBI-vulnerable"), and some never recovered ("chronically-impaired"). Three clinically relevant treatments for impulse-control or TBI, amphetamine, atomoxetine, and amantadine, were assessed for efficacy in treating injury-induced deficits. Susceptibility to TBI affected the response to pharmacological challenge with amphetamine. Whereas sham and TBI-resilient animals showed characteristic impairments in impulse control at higher doses, amphetamine had the opposite effect in chronically impaired rats, improving task performance. In contrast, atomoxetine and amantadine reduced premature responding but increased omissions, suggesting psychomotor slowing. Analysis of brain tissue revealed that generalized neuroinflammation was associated with impulsivity even when accounting for the degree of brain damage. This is one of the first studies to characterize psychiatric-like symptoms in experimental TBI. Our data highlight the importance of testing pharmacotherapies in TBI models in order to predict efficacy, and suggest that neuroinflammation may represent a treatment target for impulse control problems following injury.
Keywords: Controlled cortical impact; amphetamine; cytokine; impulsivity; prelimbic.
Figures
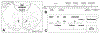




References
-
- Diaz-Arrastia R, and Kenney K (2014) Epidemiology of traumatic brain injury, in Traumatic Brain Injury (Vos P, and Diaz-Arrastia R, Eds.) pp 183–191, Wiley-Blackwell, Oxford, UK.
-
- Rosenbaum SB, and Lipton ML (2012) Embracing chaos: The scope and importance of clinical and pathological heterogeneity in mTBI. Brain Imaging and Behavior 6, 255–282. - PubMed
-
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, and Sniezek JE (1999) Traumatic brain injury in the United States: A public health perspective. Journal of Head Trauma Rehabilitation 14, 602–615. - PubMed
-
- Zaloshnja E, Miller T, Langlois JA, and Selassie AW (2008) Prevalence of Long-term disability from traumatic brain injury in the civilian population of the United States, 2005. The Journal of Head Trauma Rehabilitation 23, 394–400. - PubMed
-
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, and Breitner JCS (2000) Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 55, 1158–1166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

