Mechanism of hard-nanomaterial clearance by the liver
- PMID: 27525571
- PMCID: PMC5132626
- DOI: 10.1038/nmat4718
Mechanism of hard-nanomaterial clearance by the liver
Abstract
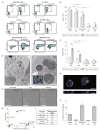
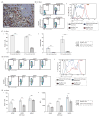
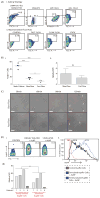
The liver and spleen are major biological barriers to translating nanomedicines because they sequester the majority of administered nanomaterials and prevent delivery to diseased tissue. Here we examined the blood clearance mechanism of administered hard nanomaterials in relation to blood flow dynamics, organ microarchitecture and cellular phenotype. We found that nanomaterial velocity reduces 1,000-fold as they enter and traverse the liver, leading to 7.5 times more nanomaterial interaction with hepatic cells relative to peripheral cells. In the liver, Kupffer cells (84.8 ± 6.4%), hepatic B cells (81.5 ± 9.3%) and liver sinusoidal endothelial cells (64.6 ± 13.7%) interacted with administered PEGylated quantum dots, but splenic macrophages took up less material (25.4 ± 10.1%) due to differences in phenotype. The uptake patterns were similar for two other nanomaterial types and five different surface chemistries. Potential new strategies to overcome off-target nanomaterial accumulation may involve manipulating intra-organ flow dynamics and modulating the cellular phenotype to alter hepatic cell interactions.
Figures



Comment in
-
Liver: Clearance of nanomaterials in the liver.Nat Rev Gastroenterol Hepatol. 2016 Oct;13(10):560. doi: 10.1038/nrgastro.2016.136. Epub 2016 Aug 24. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27555473 No abstract available.
References
-
- Kim BY, Rutka JT, Chan WC. Nanomedicine. New Engl J Med. 2010;363:2434–2443. - PubMed
-
- Nasongkla N, et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427–2430. - PubMed
-
- Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2002;21:47–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

