3D-Printing for Analytical Ultracentrifugation
- PMID: 27525659
- PMCID: PMC4985148
- DOI: 10.1371/journal.pone.0155201
3D-Printing for Analytical Ultracentrifugation
Abstract
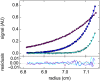
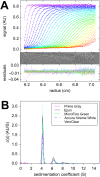
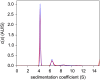
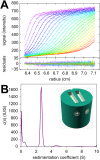
Analytical ultracentrifugation (AUC) is a classical technique of physical biochemistry providing information on size, shape, and interactions of macromolecules from the analysis of their migration in centrifugal fields while free in solution. A key mechanical element in AUC is the centerpiece, a component of the sample cell assembly that is mounted between the optical windows to allow imaging and to seal the sample solution column against high vacuum while exposed to gravitational forces in excess of 300,000 g. For sedimentation velocity it needs to be precisely sector-shaped to allow unimpeded radial macromolecular migration. During the history of AUC a great variety of centerpiece designs have been developed for different types of experiments. Here, we report that centerpieces can now be readily fabricated by 3D printing at low cost, from a variety of materials, and with customized designs. The new centerpieces can exhibit sufficient mechanical stability to withstand the gravitational forces at the highest rotor speeds and be sufficiently precise for sedimentation equilibrium and sedimentation velocity experiments. Sedimentation velocity experiments with bovine serum albumin as a reference molecule in 3D printed centerpieces with standard double-sector design result in sedimentation boundaries virtually indistinguishable from those in commercial double-sector epoxy centerpieces, with sedimentation coefficients well within the range of published values. The statistical error of the measurement is slightly above that obtained with commercial epoxy, but still below 1%. Facilitated by modern open-source design and fabrication paradigms, we believe 3D printed centerpieces and AUC accessories can spawn a variety of improvements in AUC experimental design, efficiency and resource allocation.
Conflict of interest statement
Figures


References
-
- Svedberg T, Rinde H. The ultra-centrifuge, a new instrument for the determination of size and distribution of size of particle in amicroscopic colloids. J Am Chem Soc. 1924;46(1923):2677–2693. 10.1021/ja01677a011 - DOI
-
- Svedberg T, Pedersen KO. The Ultracentrifuge. London: Oxford University Press; 1940.
-
- Schachman HK. Ultracentrifugation in Biochemistry. New York: Academic Press; 1959.
-
- Scott DJ, Harding SE, Rowe AJ. Modern Analytical Ultracentrifugation: Techniques and Methods. Cambridge: The Royal Society of Chemistry; 2005.
-
- Schuck P, Zhao H, Brautigam CA, Ghirlando R. Basic Principles of Analytical Ultracentrifugation. Boca Raton, FL: CRC Press; 2015.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

