Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion
- PMID: 27526711
- PMCID: PMC4995078
- DOI: 10.1084/jem.20151563
Exogenous TNFR2 activation protects from acute GvHD via host T reg cell expansion
Abstract
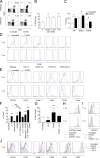
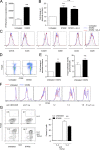
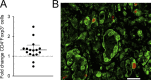
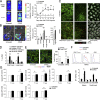
Donor CD4(+)Foxp3(+) regulatory T cells (T reg cells) suppress graft-versus-host disease (GvHD) after allogeneic hematopoietic stem cell transplantation (HCT [allo-HCT]). Current clinical study protocols rely on the ex vivo expansion of donor T reg cells and their infusion in high numbers. In this study, we present a novel strategy for inhibiting GvHD that is based on the in vivo expansion of recipient T reg cells before allo-HCT, exploiting the crucial role of tumor necrosis factor receptor 2 (TNFR2) in T reg cell biology. Expanding radiation-resistant host T reg cells in recipient mice using a mouse TNFR2-selective agonist before allo-HCT significantly prolonged survival and reduced GvHD severity in a TNFR2- and T reg cell-dependent manner. The beneficial effects of transplanted T cells against leukemia cells and infectious pathogens remained unaffected. A corresponding human TNFR2-specific agonist expanded human T reg cells in vitro. These observations indicate the potential of our strategy to protect allo-HCT patients from acute GvHD by expanding T reg cells via selective TNFR2 activation in vivo.
© 2016 Chopra et al.
Figures




Similar articles
-
[Influence of donor T(reg) cells on GVHD and hematopoietic reconstitution after allogeneic bone marrow transplantation in mice].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007 Jun;15(3):547-52. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2007. PMID: 17605863 Chinese.
-
Control of GVHD by regulatory T cells depends on TNF produced by T cells and TNFR2 expressed by regulatory T cells.Blood. 2016 Sep 22;128(12):1651-9. doi: 10.1182/blood-2016-02-700849. Epub 2016 Aug 9. Blood. 2016. PMID: 27506541
-
Graft-versus-leukemia and graft-versus-host reactions after donor lymphocyte infusion are initiated by host-type antigen-presenting cells and regulated by regulatory T cells in early and long-term chimeras.Biol Blood Marrow Transplant. 2006 Apr;12(4):397-407. doi: 10.1016/j.bbmt.2005.11.519. Biol Blood Marrow Transplant. 2006. PMID: 16545723
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
CD4+CD25+ regulatory T cells in human hematopoietic cell transplantation.Semin Cancer Biol. 2006 Apr;16(2):150-9. doi: 10.1016/j.semcancer.2005.11.008. Epub 2006 Jan 23. Semin Cancer Biol. 2006. PMID: 16431128 Review.
Cited by
-
Generic design principles for antibody-based tumour necrosis factor (TNF) receptor 2 (TNFR2) agonists with FcγR-independent agonism.Theranostics. 2024 Jan 1;14(2):496-509. doi: 10.7150/thno.84404. eCollection 2024. Theranostics. 2024. PMID: 38169605 Free PMC article.
-
TNFR2 ligation in human T regulatory cells enhances IL2-induced cell proliferation through the non-canonical NF-κB pathway.Sci Rep. 2018 Aug 13;8(1):12079. doi: 10.1038/s41598-018-30621-4. Sci Rep. 2018. PMID: 30104686 Free PMC article.
-
Emerging translational strategies and challenges for enhancing regulatory T cell therapy for graft-versus-host disease.Front Immunol. 2022 Jul 28;13:926550. doi: 10.3389/fimmu.2022.926550. eCollection 2022. Front Immunol. 2022. PMID: 35967386 Free PMC article. Review.
-
Current approaches to prevent and treat GVHD after allogeneic stem cell transplantation.Hematology Am Soc Hematol Educ Program. 2018 Nov 30;2018(1):228-235. doi: 10.1182/asheducation-2018.1.228. Hematology Am Soc Hematol Educ Program. 2018. PMID: 30504315 Free PMC article. Review.
-
Tumor Necrosis Factor and Regulatory T Cells.Yonsei Med J. 2019 Feb;60(2):126-131. doi: 10.3349/ymj.2019.60.2.126. Yonsei Med J. 2019. PMID: 30666833 Free PMC article. Review.
References
-
- Beilhack A., Schulz S., Baker J., Beilhack G.F., Wieland C.B., Herman E.I., Baker E.M., Cao Y.A., Contag C.H., and Negrin R.S.. 2005. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 106:1113–1122. 10.1182/blood-2005-02-0509 - DOI - PMC - PubMed
-
- Beilhack A., Schulz S., Baker J., Beilhack G.F., Nishimura R., Baker E.M., Landan G., Herman E.I., Butcher E.C., Contag C.H., and Negrin R.S.. 2008. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 111:2919–2928. 10.1182/blood-2007-09-112789 - DOI - PMC - PubMed
-
- Brunstein C.G., Miller J.S., Cao Q., McKenna D.H., Hippen K.L., Curtsinger J., Defor T., Levine B.L., June C.H., Rubinstein P., et al. . 2011. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 117:1061–1070. 10.1182/blood-2010-07-293795 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

