Astrocytic Acid-Sensing Ion Channel 1a Contributes to the Development of Chronic Epileptogenesis
- PMID: 27526777
- PMCID: PMC4985693
- DOI: 10.1038/srep31581
Astrocytic Acid-Sensing Ion Channel 1a Contributes to the Development of Chronic Epileptogenesis
Erratum in
-
Corrigendum: Astrocytic Acid-Sensing Ion Channel 1a Contributes to the Development of Chronic Epileptogenesis.Sci Rep. 2016 Dec 6;6:38593. doi: 10.1038/srep38593. Sci Rep. 2016. PMID: 27922108 Free PMC article. No abstract available.
Abstract
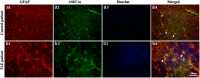
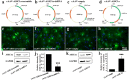
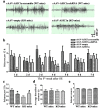
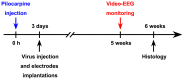
Unraveling mechanisms underlying epileptogenesis after brain injury is an unmet medical challenge. Although histopathological studies have revealed that reactive astrogliosis and tissue acidosis are prominent features in epileptogenic foci, their roles in epileptogenesis remain unclear. Here, we explored whether astrocytic acid-sensing ion channel-1a (ASIC1a) contributes to the development of chronic epilepsy. High levels of ASIC1a were measured in reactive astrocytes in the hippocampi of patients with temporal lobe epilepsy (TLE) and epileptic mice. Extracellular acidosis caused a significant Ca(2+) influx in cultured astrocytes, and this influx was sensitive to inhibition by the ASIC1a-specific blocker psalmotoxin 1 (PcTX1). In addition, recombinant adeno-associated virus (rAAV) vectors carrying a GFAP promoter in conjunction with ASIC1a shRNA or cDNA were generated to suppress or restore, respectively, ASIC1a expression in astrocytes. Injection of rAAV-ASIC1a-shRNA into the dentate gyrus of the wide type TLE mouse model resulted in the inhibition of astrocytic ASIC1a expression and a reduction in spontaneous seizures. By contrast, rAAV-ASIC1a-cDNA restored astrocytic ASIC1a expression in an ASIC1a knock-out TLE mouse model and increased the frequency of spontaneous seizures. Taken together, our results reveal that astrocytic ASIC1a may be an attractive new target for the treatment of epilepsy.
Figures




References
-
- Pitkanen A. & Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol 10, 173–186 (2011). - PubMed
-
- Franco V., French J. A. & Perucca E. Challenges in the clinical development of new antiepileptic drugs. Pharmacol Res, 103, 95–104 (2015). - PubMed
-
- Hubbard J. A., Hsu M. S., Fiacco T. A. & Binder D. K. Glial cell changes in epilepsy: overview of the clinical problem and therapeutic opportunities. Neurochem Int 63, 638–651 (2013). - PubMed
-
- Sendrowski K. & Sobaniec W. Hippocampus, hippocampal sclerosis and epilepsy. Pharmacol Rep 65, 555–565 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

