The Ribonuclease A Superfamily in Humans: Canonical RNases as the Buttress of Innate Immunity
- PMID: 27527162
- PMCID: PMC5000675
- DOI: 10.3390/ijms17081278
The Ribonuclease A Superfamily in Humans: Canonical RNases as the Buttress of Innate Immunity
Abstract
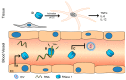
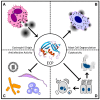
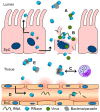
In humans, the ribonuclease A (RNase A) superfamily contains eight different members that have RNase activities, and all of these members are encoded on chromosome 14. The proteins are secreted by a large variety of different tissues and cells; however, a comprehensive understanding of these proteins' physiological roles is lacking. Different biological effects can be attributed to each protein, including antiviral, antibacterial and antifungal activities as well as cytotoxic effects against host cells and parasites. Different immunomodulatory effects have also been demonstrated. This review summarizes the available data on the human RNase A superfamily and illustrates the significant role of the eight canonical RNases in inflammation and the host defence system against infections.
Keywords: antimicrobial activity; canonical RNases; host defence protein; human RNases; secreted RNases.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

