Neutrons describe ectoine effects on water H-bonding and hydration around a soluble protein and a cell membrane
- PMID: 27527336
- PMCID: PMC4985633
- DOI: 10.1038/srep31434
Neutrons describe ectoine effects on water H-bonding and hydration around a soluble protein and a cell membrane
Abstract
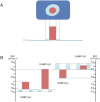
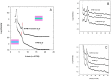
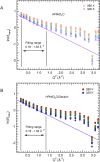
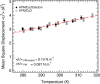
Understanding adaptation to extreme environments remains a challenge of high biotechnological potential for fundamental molecular biology. The cytosol of many microorganisms, isolated from saline environments, reversibly accumulates molar concentrations of the osmolyte ectoine to counterbalance fluctuating external salt concentrations. Although they have been studied extensively by thermodynamic and spectroscopic methods, direct experimental structural data have, so far, been lacking on ectoine-water-protein interactions. In this paper, in vivo deuterium labeling, small angle neutron scattering, neutron membrane diffraction and inelastic scattering are combined with neutron liquids diffraction to characterize the extreme ectoine-containing solvent and its effects on purple membrane of H. salinarum and E. coli maltose binding protein. The data reveal that ectoine is excluded from the hydration layer at the membrane surface and does not affect membrane molecular dynamics, and prove a previous hypothesis that ectoine is excluded from a monolayer of dense hydration water around the soluble protein. Neutron liquids diffraction to atomic resolution shows how ectoine enhances the remarkable properties of H-bonds in water-properties that are essential for the proper organization, stabilization and dynamics of biological structures.
Figures

References
-
- Severin J., Wohlfarth A. & Galinski E. A. The Predominant Role Of Recently Discovered Tetrahydropyrimidines For The Osmoadaptation Of Halophilic Eubacteria. Journal of General Microbiology 138, 1629–1638 (1992).
-
- Vreeland R. H., Litchfield C. D., Martin E. L. & Elliot E. Halomonas-Elongata, A New Genus And Species Of Extremely Salt-Tolerant Bacteria. International Journal of Systematic Bacteriology 30, 485–495 (1980).
-
- Quillaguaman J., Hatti-Kaul R., Mattiasson B., Alvarez M. T. & Delgado O. Halomonas boliviensis sp nov., an alkalitolerant, moderate halophile isolated from soil around a Bolivian hypersaline lake. International Journal of Systematic and Evolutionary Microbiology 54, 721–725, doi: 10.1099/ijs.0.02800-0 (2004). - DOI - PubMed
-
- da Costa M. S., Santos H. & Galinski E. A. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Advances in biochemical engineering/biotechnology 61, 117–153 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

