The Role of Additional Sex Combs-Like Proteins in Cancer
- PMID: 27527698
- PMCID: PMC5046687
- DOI: 10.1101/cshperspect.a026526
The Role of Additional Sex Combs-Like Proteins in Cancer
Abstract
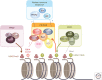
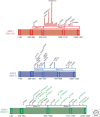
Additional sex combs-like (ASXL) proteins are mammalian homologs of Addition of sex combs (Asx), a protein that regulates the balance of trithorax and Polycomb function in Drosophila. All three ASXL family members (ASXL1, ASXL2, and ASXL3) are affected by somatic or de novo germline mutations in cancer or rare developmental syndromes, respectively. Although Asx is characterized as a catalytic partner for the deubiquitinase Calypso (or BAP1), there are domains of ASXL proteins that are distinct from Asx and the roles and redundancies of ASXL members are not yet well understood. Moreover, it is not yet fully clarified if commonly encountered ASXL1 mutations result in a loss of protein or stable expression of a truncated protein with dominant-negative or gain-of-function properties. This review summarizes our current knowledge of the biological and functional roles of ASXL members in development, cancer, and transcription.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Avila M, Kirchhoff M, Marle N, Hove HD, Chouchane M, Thauvin-Robinet C, Masurel A, Mosca-Boidron AL, Callier P, Mugneret F, et al. 2013. Delineation of a new chromosome 20q11.2 duplication syndrome including the ASXL1 gene. Am J Med Genet A 161: 1594–1598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
