Binding site elucidation and structure guided design of macrocyclic IL-17A antagonists
- PMID: 27527709
- PMCID: PMC4985813
- DOI: 10.1038/srep30859
Binding site elucidation and structure guided design of macrocyclic IL-17A antagonists
Abstract
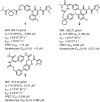
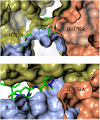
Interleukin-17A (IL-17A) is a principal driver of multiple inflammatory and immune disorders. Antibodies that neutralize IL-17A or its receptor (IL-17RA) deliver efficacy in autoimmune diseases, but no small-molecule IL-17A antagonists have yet progressed into clinical trials. Investigation of a series of linear peptide ligands to IL-17A and characterization of their binding site has enabled the design of novel macrocyclic ligands that are themselves potent IL-17A antagonists.
Figures


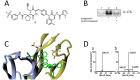

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

