Inactivated porcine reproductive and respiratory syndrome virus vaccine adjuvanted with Montanide™ Gel 01 ST elicits virus-specific cross-protective inter-genotypic response in piglets
- PMID: 27527768
- PMCID: PMC7111292
- DOI: 10.1016/j.vetmic.2016.06.014
Inactivated porcine reproductive and respiratory syndrome virus vaccine adjuvanted with Montanide™ Gel 01 ST elicits virus-specific cross-protective inter-genotypic response in piglets
Abstract
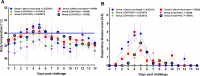
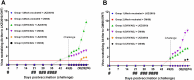
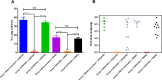
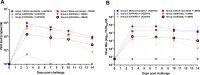
The efficacy of a novel BEI-inactivated porcine reproductive and respiratory syndrome virus (PRRSV) candidate vaccine in pigs, developed at RIBSP Republic of Kazakhstan and delivered with an adjuvant Montanide™ Gel 01 ST (D/KV/ADJ) was compared with a commercial killed PRRSV vaccine (NVDC-JXA1, C/KV/ADJ) used widely in swine herds of the Republic of Kazakhstan. Clinical parameters (body temperature and respiratory disease scores), virological and immunological profiles [ELISA and virus neutralizing (VN) antibody titers], macroscopic lung lesions and viral load in the lungs (quantitative real-time PCR and cell culture assay) were assessed in vaccinated and both genotype 1 and 2 PRRSV challenged pigs. Our results showed that the commercial vaccine failed to protect pigs adequately against the clinical disease, viremia and lung lesions caused by the challenged field isolates, Kazakh strains of PRRSV type 1 and type 2 genotypes. In contrast, clinical protection, absence of viremia and lung lesions in D/KV/ADJ vaccinated pigs was associated with generation of VN antibodies in both homologous vaccine strain LKZ/2010 (PRRSV type 2) and a heterogeneous type 1 PRRSV strain (CM/08) challenged pigs. Thus, our data indicated the induction of cross-protective VN antibodies by D/KV/ADJ vaccine, and importantly demonstrated that an inactivated PRRSV vaccine could also induce cross-protective response across the viral genotype.
Keywords: Binary ethylenimine; Cross-protection; Killed vaccines; Neutralizing antibodies; PRRSV; Polymeric adjuvant.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Antibody response and maternal immunity upon boosting PRRSV-immune sows with experimental farm-specific and commercial PRRSV vaccines.Vet Microbiol. 2013 Dec 27;167(3-4):260-71. doi: 10.1016/j.vetmic.2013.08.017. Epub 2013 Aug 28. Vet Microbiol. 2013. PMID: 24041768
-
An interferon inducing porcine reproductive and respiratory syndrome virus vaccine candidate elicits protection against challenge with the heterologous virulent type 2 strain VR-2385 in pigs.Vaccine. 2017 Jan 3;35(1):125-131. doi: 10.1016/j.vaccine.2016.11.020. Epub 2016 Nov 18. Vaccine. 2017. PMID: 27876202
-
Development of an experimental inactivated PRRSV vaccine that induces virus-neutralizing antibodies.Vet Res. 2009 Nov-Dec;40(6):63. doi: 10.1051/vetres/2009046. Epub 2009 Aug 13. Vet Res. 2009. PMID: 19674539 Clinical Trial.
-
Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction.Vaccine. 2015 Jun 17;33(27):3065-72. doi: 10.1016/j.vaccine.2015.04.102. Epub 2015 May 14. Vaccine. 2015. PMID: 25980425 Review.
-
Vectored vaccines to protect against PRRSV.Virus Res. 2010 Dec;154(1-2):150-60. doi: 10.1016/j.virusres.2010.06.017. Epub 2010 Jun 25. Virus Res. 2010. PMID: 20600388 Free PMC article. Review.
Cited by
-
Safety and long-lasting immunity of the combined administration of a modified-live virus vaccine against porcine reproductive and respiratory syndrome virus 1 and an inactivated vaccine against porcine parvovirus and Erysipelothrix rhusiopathiae in breeding pigs.Porcine Health Manag. 2019 Apr 25;5:11. doi: 10.1186/s40813-019-0118-9. eCollection 2019. Porcine Health Manag. 2019. PMID: 31057805 Free PMC article.
-
Oral Supplementation of Houttuynia cordata Extract Reduces Viremia in PRRSV-1 Modified-Live Virus-Vaccinated Pigs in Response to the HP-PRRSV-2 Challenge.Front Immunol. 2022 Jul 18;13:929338. doi: 10.3389/fimmu.2022.929338. eCollection 2022. Front Immunol. 2022. PMID: 35924249 Free PMC article.
-
Coinfections and Phenotypic Antimicrobial Resistance in Actinobacillus pleuropneumoniae Strains Isolated From Diseased Swine in North Western Germany-Temporal Patterns in Samples From Routine Laboratory Practice From 2006 to 2020.Front Vet Sci. 2022 Jan 28;8:802570. doi: 10.3389/fvets.2021.802570. eCollection 2021. Front Vet Sci. 2022. PMID: 35155648 Free PMC article.
-
Development and Characterization of a Recombinant galT-galU Protein for Broad-Spectrum Immunoprotection Against Porcine Contagious Pleuropneumonia.Int J Mol Sci. 2025 Apr 11;26(8):3634. doi: 10.3390/ijms26083634. Int J Mol Sci. 2025. PMID: 40332240 Free PMC article.
-
Long-Term Protection Elicited by an Inactivated Vaccine Supplemented with a Water-Based Adjuvant against Infectious Hematopoietic Necrosis Virus in Rainbow Trout (Oncorhynchus mykiss).Microbiol Spectr. 2022 Dec 21;10(6):e0324522. doi: 10.1128/spectrum.03245-22. Epub 2022 Nov 21. Microbiol Spectr. 2022. PMID: 36409094 Free PMC article.
References
-
- Azanbekova М.А., Mambetaliyev M., Azhibayev A.Z., Tabynov K.K., Khussainov D.M. Selection of effective activation agents for porcine reproductive and respiratory syndrome virus. Veterinary. 2014;2:50–55. (in Russian)
-
- Binjawadagi B., Dwivedi V., Manickam C., Ouyang K., Torrelles J.B., Renukaradhya G.J. An innovative approach to induce cross-protective immunity against porcine reproductive and respiratory syndrome virus in the lungs of pigs through adjuvanted nanotechnology-based vaccination. Int. J. Nanomed. 2014;9:1519–1535. - PMC - PubMed
-
- Binjawadagi B., Dwivedi V., Manickam C., Ouyang K., Wu Y., Lee L.J., Torrelles J.B., Renukaradhya G.J. Adjuvanted poly(lactic-co-glycolic) acid nanoparticle-entrapped inactivated porcine reproductive and respiratory syndrome virus vaccine elicits cross-protective immune response in pigs. Int. J. Nanomed. 2014;9:679–694. - PMC - PubMed
-
- Botner A., Strandbygaard B., Sorensen K.J., Have P., Madsen K.G., Madsen E.S., Alexandersen S. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet. Rec. 1997;141:497–499. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

