Inactivated porcine reproductive and respiratory syndrome virus vaccine adjuvanted with Montanide™ Gel 01 ST elicits virus-specific cross-protective inter-genotypic response in piglets
- PMID: 27527768
- PMCID: PMC7111292
- DOI: 10.1016/j.vetmic.2016.06.014
Inactivated porcine reproductive and respiratory syndrome virus vaccine adjuvanted with Montanide™ Gel 01 ST elicits virus-specific cross-protective inter-genotypic response in piglets
Abstract
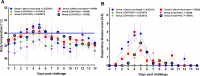
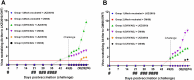
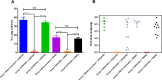
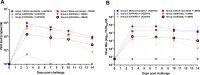
The efficacy of a novel BEI-inactivated porcine reproductive and respiratory syndrome virus (PRRSV) candidate vaccine in pigs, developed at RIBSP Republic of Kazakhstan and delivered with an adjuvant Montanide™ Gel 01 ST (D/KV/ADJ) was compared with a commercial killed PRRSV vaccine (NVDC-JXA1, C/KV/ADJ) used widely in swine herds of the Republic of Kazakhstan. Clinical parameters (body temperature and respiratory disease scores), virological and immunological profiles [ELISA and virus neutralizing (VN) antibody titers], macroscopic lung lesions and viral load in the lungs (quantitative real-time PCR and cell culture assay) were assessed in vaccinated and both genotype 1 and 2 PRRSV challenged pigs. Our results showed that the commercial vaccine failed to protect pigs adequately against the clinical disease, viremia and lung lesions caused by the challenged field isolates, Kazakh strains of PRRSV type 1 and type 2 genotypes. In contrast, clinical protection, absence of viremia and lung lesions in D/KV/ADJ vaccinated pigs was associated with generation of VN antibodies in both homologous vaccine strain LKZ/2010 (PRRSV type 2) and a heterogeneous type 1 PRRSV strain (CM/08) challenged pigs. Thus, our data indicated the induction of cross-protective VN antibodies by D/KV/ADJ vaccine, and importantly demonstrated that an inactivated PRRSV vaccine could also induce cross-protective response across the viral genotype.
Keywords: Binary ethylenimine; Cross-protection; Killed vaccines; Neutralizing antibodies; PRRSV; Polymeric adjuvant.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures

References
-
- Azanbekova М.А., Mambetaliyev M., Azhibayev A.Z., Tabynov K.K., Khussainov D.M. Selection of effective activation agents for porcine reproductive and respiratory syndrome virus. Veterinary. 2014;2:50–55. (in Russian)
-
- Binjawadagi B., Dwivedi V., Manickam C., Ouyang K., Torrelles J.B., Renukaradhya G.J. An innovative approach to induce cross-protective immunity against porcine reproductive and respiratory syndrome virus in the lungs of pigs through adjuvanted nanotechnology-based vaccination. Int. J. Nanomed. 2014;9:1519–1535. - PMC - PubMed
-
- Binjawadagi B., Dwivedi V., Manickam C., Ouyang K., Wu Y., Lee L.J., Torrelles J.B., Renukaradhya G.J. Adjuvanted poly(lactic-co-glycolic) acid nanoparticle-entrapped inactivated porcine reproductive and respiratory syndrome virus vaccine elicits cross-protective immune response in pigs. Int. J. Nanomed. 2014;9:679–694. - PMC - PubMed
-
- Botner A., Strandbygaard B., Sorensen K.J., Have P., Madsen K.G., Madsen E.S., Alexandersen S. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet. Rec. 1997;141:497–499. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

