Phosphatidylinositide 3-kinase (PI3K) and PI3K-related kinase (PIKK) activity contributes to radioresistance in thyroid carcinomas
- PMID: 27527858
- PMCID: PMC5325350
- DOI: 10.18632/oncotarget.11056
Phosphatidylinositide 3-kinase (PI3K) and PI3K-related kinase (PIKK) activity contributes to radioresistance in thyroid carcinomas
Abstract
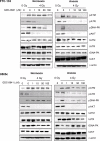
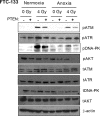
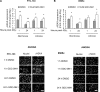
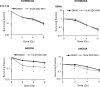
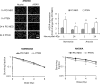
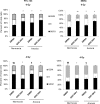
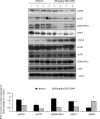
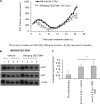
Anaplastic (ATC) and certain follicular thyroid-carcinomas (FTCs) are radioresistant. The Phosphatidylinositide 3-kinase (PI3K) pathway is commonly hyperactivated in thyroid-carcinomas. PI3K can modify the PI3K-related kinases (PIKKs) in response to radiation: How PIKKs interact with PI3K and contribute to radioresistance in thyroid-carcinomas is unknown. Further uncertainties exist in how these interactions function under the radioresistant hypoxic microenvironment. Under normoxia/anoxia, ATC (8505c) and FTC (FTC-133) cells were irradiated, with PI3K-inhibition (via GDC-0941 and PTEN-reconstitution into PTEN-null FTC-133s) and effects on PIKK-activation, DNA-damage, clonogenic-survival and cell cycle, assessed. FTC-xenografts were treated with 5 × 2 Gy, ± 50 mg/kg GDC-0941 (twice-daily; orally) for 14 days and PIKK-activation and tumour-growth assessed. PIKK-expression was additionally assessed in 12 human papillary thyroid-carcinomas, 13 FTCs and 12 ATCs. GDC-0941 inhibited radiation-induced activation of Ataxia-telangiectasia mutated (ATM), ATM-and Rad3-related (ATR) and DNA-dependent protein kinase catalytic subunit (DNA-PKcs). Inhibition of ATM and DNA-PKcs was PI3K-dependent, since activation was reduced in PTEN-reconstituted FTC-133s. Inhibition of PIKK-activation was greater under anoxia: Consequently, whilst DNA-damage was increased and prolonged under both normoxia and anoxia, PI3K-inhibition only reduced clonogenic-survival under anoxia. GDC-0941 abrogated radiation-induced cell cycle arrest, an effect most likely linked to the marked inhibition of ATR-activation. Importantly, GDC-0941 inhibited radiation-induced PIKK-activation in FTC-xenografts leading to a significant increase in time taken for tumours to triple in size: 26.5 ± 5 days (radiation-alone) versus 31.5 ± 5 days (dual-treatment). PIKKs were highly expressed across human thyroid-carcinoma classifications, with ATM scoring consistently lower. Interestingly, some loss of ATM and DNA-PKcs was observed. These data provide new insight into the mechanisms of hypoxia-associated radioresistance in thyroid-carcinoma.
Keywords: ATM; ATR; DNA-PKcs; PI3K; radioresistance.
Conflict of interest statement
The authors have nothing to disclose.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

