A single nucleotide polymorphism genotyping platform for the authentication of patient derived xenografts
- PMID: 27528024
- PMCID: PMC5312397
- DOI: 10.18632/oncotarget.11125
A single nucleotide polymorphism genotyping platform for the authentication of patient derived xenografts
Abstract
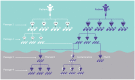
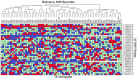
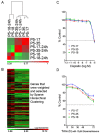
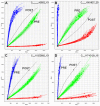
Patient derived xenografts (PDXs) have become a vital, frequently used, component of anti-cancer drug development. PDXs can be serially passaged in vivo for years, and shared across laboratories. As a consequence, the potential for mis-identification and cross-contamination is possible, yet authentication of PDXs appears limited. We present a PDX Authentication System (PAS), by combining a commercially available OpenArray assay of single nucleotide polymorphisms (SNPs) with in-house R studio programs, to validate PDXs established in individual mice from acute lymphoblastic leukemia biopsies. The PAS is sufficiently robust to identify contamination at levels as low as 3%, similar to the gold standard of short tandem repeat (STR) profiling. We have surveyed a panel of PDXs established from 73 individual leukemia patients, and found that the PAS provided sufficient discriminatory power to identify each xenograft. The identified SNP-discrepant PDXs demonstrated distinct gene expression profiles, indicating a risk of contamination for PDXs at high passage number. The PAS also allows for the authentication of tumor cells with complex karyotypes from solid tumors including prostate cancer and Ewing's sarcoma. This study highlights the demands of authenticating PDXs for cancer research, and evaluates a reliable authentication platform that utilizes a commercially available and cost-effective system.
Keywords: OpenArray; R studio; SNP genotyping; authentication; patient derived xenografts.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



Similar articles
-
Molecular heterogeneity of non-small cell lung carcinoma patient-derived xenografts closely reflect their primary tumors.Int J Cancer. 2017 Feb 1;140(3):662-673. doi: 10.1002/ijc.30472. Epub 2016 Nov 7. Int J Cancer. 2017. PMID: 27750381
-
Movember GAP1 PDX project: An international collection of serially transplantable prostate cancer patient-derived xenograft (PDX) models.Prostate. 2018 Dec;78(16):1262-1282. doi: 10.1002/pros.23701. Epub 2018 Aug 2. Prostate. 2018. PMID: 30073676
-
Patient-Derived Xenograft Models for Translational Prostate Cancer Research and Drug Development.Methods Mol Biol. 2024;2806:153-185. doi: 10.1007/978-1-0716-3858-3_12. Methods Mol Biol. 2024. PMID: 38676802
-
The future of patient-derived xenografts in prostate cancer research.Nat Rev Urol. 2023 Jun;20(6):371-384. doi: 10.1038/s41585-022-00706-x. Epub 2023 Jan 17. Nat Rev Urol. 2023. PMID: 36650259 Free PMC article. Review.
-
Genomic heterogeneity in acute leukemia.Cytogenet Genome Res. 2013;139(3):174-80. doi: 10.1159/000346797. Epub 2013 Jan 26. Cytogenet Genome Res. 2013. PMID: 23363651 Review.
Cited by
-
Reversal of glucocorticoid resistance in paediatric acute lymphoblastic leukaemia is dependent on restoring BIM expression.Br J Cancer. 2020 Jun;122(12):1769-1781. doi: 10.1038/s41416-020-0824-8. Epub 2020 Apr 3. Br J Cancer. 2020. PMID: 32242100 Free PMC article.
-
The MURAL collection of prostate cancer patient-derived xenografts enables discovery through preclinical models of uro-oncology.Nat Commun. 2021 Aug 19;12(1):5049. doi: 10.1038/s41467-021-25175-5. Nat Commun. 2021. PMID: 34413304 Free PMC article.
-
Authentication, characterization and contamination detection of cell lines, xenografts and organoids by barcode deep NGS sequencing.NAR Genom Bioinform. 2020 Aug 18;2(3):lqaa060. doi: 10.1093/nargab/lqaa060. eCollection 2020 Sep. NAR Genom Bioinform. 2020. PMID: 33575611 Free PMC article.
-
The Molecular Subtype of Adult Acute Lymphoblastic Leukemia Samples Determines the Engraftment Site and Proliferation Kinetics in Patient-Derived Xenograft Models.Cells. 2022 Jan 3;11(1):150. doi: 10.3390/cells11010150. Cells. 2022. PMID: 35011712 Free PMC article.
-
Genomic Profiling of Childhood Tumor Patient-Derived Xenograft Models to Enable Rational Clinical Trial Design.Cell Rep. 2019 Nov 5;29(6):1675-1689.e9. doi: 10.1016/j.celrep.2019.09.071. Cell Rep. 2019. PMID: 31693904 Free PMC article.
References
-
- Saland E, Boutzen H, Castellano R, Pouyet L, Griessinger E, Larrue C, de Toni F, Scotland S, David M, Danet-Desnoyers G, Vergez F, Barreira Y, Collette Y, et al. A robust and rapid xenograft model to assess efficacy of chemotherapeutic agents for human acute myeloid leukemia. Blood Cancer J. 2015;5:e297. - PMC - PubMed
-
- Gould SE, Junttila MR, de Sauvage FJ. Translational value of mouse models in oncology drug development. Nat Med. 2015;21:431–439. - PubMed
-
- Estey E, Levine RL, Lowenberg B. Current challenges in clinical development of “targeted therapies”: the case of acute myeloid leukemia. Blood. 2015;125:2461–2466. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

