Pregnancy-specific glycoprotein 9 (PSG9), a driver for colorectal cancer, enhances angiogenesis via activation of SMAD4
- PMID: 27528036
- PMCID: PMC5308672
- DOI: 10.18632/oncotarget.11146
Pregnancy-specific glycoprotein 9 (PSG9), a driver for colorectal cancer, enhances angiogenesis via activation of SMAD4
Abstract
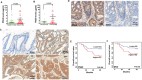
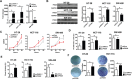
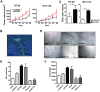
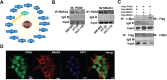
PSG9 is a member of the pregnancy-specific glycoprotein (PSG) family and has been shown to contribute to the progression of colorectal cancer (CRC) and cancer-related angiogenesis. Here, we aim to investigate abnormal PSG9 levels in patients with CRC and to emphasize the role of PSG9 in driving tumorigenesis. Serum from 140 patients with CRC and 125 healthy controls as well as 74 paired tumors and adjacent normal tissue were used to determine PSG9 levels. We discovered that PSG9 was significantly increased in serum (P<0.001) and in tumor tissues (P<0.001) from patients with CRC. Interestingly, the increased PSG9 levels correlated with poor survival (P=0.009) and microvessel density (MVD) (P=0.034). The overexpression of PSG9 strongly promoted the proliferation and migration of HCT-116 and HT-29 cells. However, PSG9 depletion inhibited the proliferation of SW-480 cells. Using a human umbilical vein endothelial cell tube-forming assay, we found that PSG9 promoted angiogenesis. The overexpression of PSG9 also increased the growth of tumor xenografts in nude mice. Co-immunoprecipitation experiments revealed that PSG9 was bound to SMAD4. The PSG9/SMAD4 complex recruited cytoplasmic SMAD2/3 to form a complex, which enhanced SMAD4 nuclear retention. The PSG9 and SMAD4 complex activated the expression of multiple angiogenesis-related genes (included IGFBP-3, PDGF-AA, GM-CSF, and VEGFA). Together, our findings illustrate the innovative mechanism by which PSG9 drives the progression of CRC and tumor angiogenesis. This occurs via nuclear translocation of PSG9/SMAD4, which activates angiogenic cytokines. Therefore, our study may provide evidence for novel treatment strategies by targeting PSG9 in antiangiogenic cancer therapy.
Keywords: PSG9; SMAD4; angiogenesis; colorectal cancer (CRC).
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures

Similar articles
-
PSG9 promotes angiogenesis by stimulating VEGFA production and is associated with poor prognosis in hepatocellular carcinoma.Sci China Life Sci. 2017 May;60(5):528-535. doi: 10.1007/s11427-016-0226-7. Epub 2017 Jan 4. Sci China Life Sci. 2017. PMID: 28078509
-
An Oncolytic Adenovirus Encoding Decorin and Granulocyte Macrophage Colony Stimulating Factor Inhibits Tumor Growth in a Colorectal Tumor Model by Targeting Pro-Tumorigenic Signals and via Immune Activation.Hum Gene Ther. 2017 Aug;28(8):667-680. doi: 10.1089/hum.2017.033. Hum Gene Ther. 2017. PMID: 28530155
-
Positive feedback loop of hepatoma-derived growth factor and β-catenin promotes carcinogenesis of colorectal cancer.Oncotarget. 2015 Oct 6;6(30):29357-74. doi: 10.18632/oncotarget.4982. Oncotarget. 2015. PMID: 26296979 Free PMC article.
-
Exploring the molecular mechanisms and therapeutic potential of SMAD4 in colorectal cancer.Cancer Biol Ther. 2024 Dec 31;25(1):2392341. doi: 10.1080/15384047.2024.2392341. Epub 2024 Aug 20. Cancer Biol Ther. 2024. PMID: 39164192 Free PMC article. Review.
-
The Role of Tumor-Associated Neutrophils in Colorectal Cancer.Int J Mol Sci. 2019 Jan 27;20(3):529. doi: 10.3390/ijms20030529. Int J Mol Sci. 2019. PMID: 30691207 Free PMC article. Review.
Cited by
-
BRAF mutations may identify a clinically distinct subset of glioblastoma.Sci Rep. 2021 Oct 8;11(1):19999. doi: 10.1038/s41598-021-99278-w. Sci Rep. 2021. PMID: 34625582 Free PMC article.
-
Multiplex fluorescent immunohistochemistry quantitatively analyses microvascular density (MVD) and the roles of TGF-β signalling in orchestrating angiogenesis in colorectal cancer.Transl Cancer Res. 2019 Apr;8(2):429-438. doi: 10.21037/tcr.2019.02.09. Transl Cancer Res. 2019. PMID: 35116775 Free PMC article.
-
Analysis of risk factors for colon cancer progression.Onco Targets Ther. 2019 May 22;12:3991-4000. doi: 10.2147/OTT.S207390. eCollection 2019. Onco Targets Ther. 2019. PMID: 31190895 Free PMC article.
-
Mechanism of ELL-associated factor 2 and vasohibin 1 regulating invasion, migration, and angiogenesis in colorectal cancer.World J Gastroenterol. 2023 Jun 28;29(24):3770-3792. doi: 10.3748/wjg.v29.i24.3770. World J Gastroenterol. 2023. PMID: 37426316 Free PMC article.
-
Targeting colorectal cancer with small-molecule inhibitors of ALDH1B1.Nat Chem Biol. 2022 Oct;18(10):1065-1075. doi: 10.1038/s41589-022-01048-w. Epub 2022 Jul 4. Nat Chem Biol. 2022. PMID: 35788181 Free PMC article.
References
-
- Moore T, Dveksler GS. Pregnancy-specific glycoproteins: complex gene families regulating maternal-fetal interactions. Int J Dev Biol. 2014;58:273–280. - PubMed
-
- Teglund S, Zhou GQ, Hammarstrom S. Characterization of cDNA encoding novel pregnancy-specific glycoprotein variants. Biochem Biophys Res Commun. 1995;211:656–664. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

