Epigenetic silencing of the NR4A3 tumor suppressor, by aberrant JAK/STAT signaling, predicts prognosis in gastric cancer
- PMID: 27528092
- PMCID: PMC4985659
- DOI: 10.1038/srep31690
Epigenetic silencing of the NR4A3 tumor suppressor, by aberrant JAK/STAT signaling, predicts prognosis in gastric cancer
Abstract
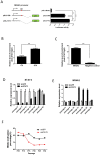
While aberrant JAK/STAT signaling is crucial to the development of gastric cancer (GC), its effects on epigenetic alterations of its transcriptional targets remains unclear. In this study, by expression microarrays coupled with bioinformatic analyses, we identified a putative STAT3 target gene, NR4A3 that was downregulated in MKN28 GC daughter cells overexpressing a constitutively activated STAT3 mutant (S16), as compared to an empty vector control (C9). Bisulphite pyrosequencing and demethylation treatment showed that NR4A3 was epigenetically silenced by promoter DNA methylation in S16 and other GC cell lines including AGS cells, showing constitutive activation of STAT3. Subsequent experiments revealed that NR4A3 promoter binding by STAT3 might repress its transcription. Long-term depletion of STAT3 derepressed NR4A3 expression, by promoter demethylation, in AGS GC cells. NR4A3 re-expression in GC cell lines sensitized the cells to cisplatin, and inhibited tumor growth in vitro and in vivo, in an animal model. Clinically, GC patients with high NR4A3 methylation, or lower NR4A3 protein expression, had significantly shorter overall survival. Intriguingly, STAT3 activation significantly associated only with NR4A3 methylation in low-stage patient samples. Taken together, aberrant JAK/STAT3 signaling epigenetically silences a potential tumor suppressor, NR4A3, in gastric cancer, plausibly representing a reliable biomarker for gastric cancer prognosis.
Figures




References
-
- Torre L. A. et al.. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108 (2015). - PubMed
-
- Lauren P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand 64, 31–49 (1965). - PubMed
-
- Kuipers E. J., Perez-Perez G. I., Meuwissen S. G. & Blaser M. J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst 87, 1777–1780 (1995). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

