Reporting of financial conflicts of interest in clinical practice guidelines: a case study analysis of guidelines from the Canadian Medical Association Infobase
- PMID: 27528247
- PMCID: PMC4986411
- DOI: 10.1186/s12913-016-1646-5
Reporting of financial conflicts of interest in clinical practice guidelines: a case study analysis of guidelines from the Canadian Medical Association Infobase
Abstract
Background: Clinical practice guidelines are widely distributed by medical associations and relied upon by physicians for the best available clinical evidence. International findings report that financial conflicts of interest (FCOI) with drug companies may influence drug recommendations and are common among guideline authors. There is no comparable study on exclusively Canadian guidelines; therefore, we provide a case study of authors' FCOI declarations in guidelines from the Canadian Medical Association (CMA) Infobase. We also assess the financial relationships between guideline-affiliated organizations and drug companies.
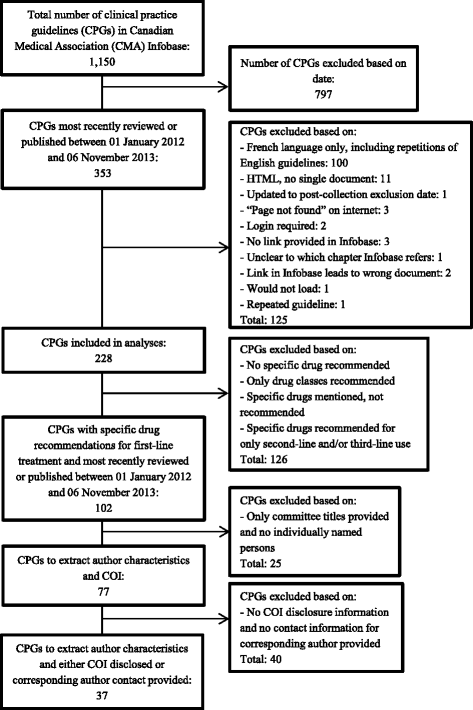
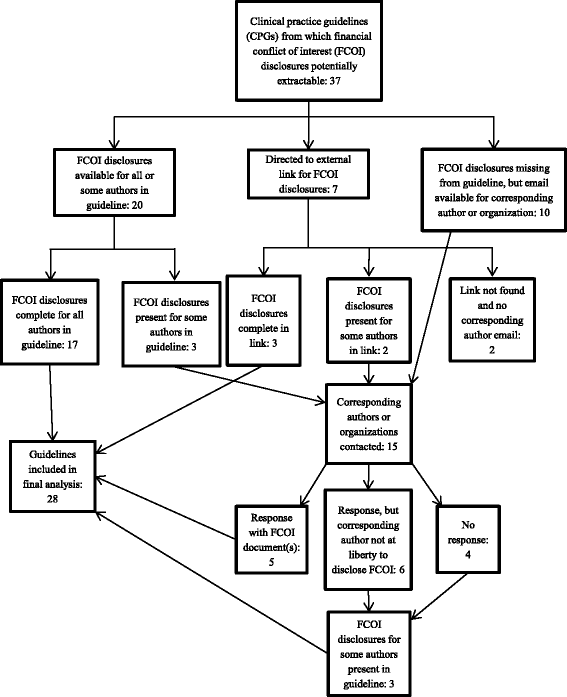
Methods: Using a population approach, we extracted first-line drug recommendations and authors' FCOI disclosures in guidelines from the CMA Infobase. We contacted the corresponding authors on guidelines when FCOI disclosures were missing for some or all authors. We also extracted guideline-affiliated organizations and searched each of their websites to determine if they had financial relationships with drug companies.
Results: We analyzed 350 authors from 28 guidelines. Authors were named on one, two, or three guidelines, yielding 400 FCOI statements. In 75.0 % of guidelines at least one author, and in 21.4 % of guidelines all authors, disclosed FCOI with drug companies. In 54.0 % of guidelines at least one author, and in 28.6 % of guidelines over half of the authors, disclosed FCOI with manufacturers of drugs that they recommended. Twenty of 48 authors on multiple guidelines reported different FCOI in their disclosures. Eight guidelines identified affiliated organizations with financial relationships with manufacturers of drugs recommended in those guidelines.
Conclusions: This is the first study to systematically describe FCOI disclosures by authors of Canadian guidelines and financial relationships between guideline-affiliated organizations and pharmaceutical companies. These financial relationships are common. Because authoritative value is assigned to guidelines distributed by medical associations, we encourage them to develop formal policies to limit the potential influence of FCOI on guideline recommendations.
Keywords: Clinical practice guidelines; Disclosure; Financial conflicts of interest; Medicine and the pharmaceutical industry; Treatment recommendations.
Figures
Similar articles
-
Financial Conflicts of Interest in Clinical Practice Guidelines: A Systematic Review.Mayo Clin Proc Innov Qual Outcomes. 2021 Jan 19;5(2):466-475. doi: 10.1016/j.mayocpiqo.2020.09.016. eCollection 2021 Apr. Mayo Clin Proc Innov Qual Outcomes. 2021. PMID: 33997642 Free PMC article. Review.
-
Financial Conflicts of Interest in Inflammatory Bowel Disease Guidelines.Inflamm Bowel Dis. 2019 Mar 14;25(4):642-645. doi: 10.1093/ibd/izy315. Inflamm Bowel Dis. 2019. PMID: 30295831
-
Evaluation of Pharmaceutical Company Payments and Conflict of Interest Disclosures Among Oncology Clinical Practice Guideline Authors in Japan.JAMA Netw Open. 2019 Apr 5;2(4):e192834. doi: 10.1001/jamanetworkopen.2019.2834. JAMA Netw Open. 2019. PMID: 31026027 Free PMC article.
-
Reporting of financial conflicts of interest by Canadian clinical practice guideline producers: a descriptive study.CMAJ. 2020 Jun 8;192(23):E617-E625. doi: 10.1503/cmaj.191737. CMAJ. 2020. PMID: 32538799 Free PMC article.
-
Authors' self-declared financial conflicts of interest do not impact the results of major cardiovascular trials.J Am Coll Cardiol. 2013 Mar 19;61(11):1137-43. doi: 10.1016/j.jacc.2012.10.056. Epub 2013 Feb 6. J Am Coll Cardiol. 2013. PMID: 23395075 Review.
Cited by
-
Are Corporations Re-Defining Illness and Health? The Diabetes Epidemic, Goal Numbers, and Blockbuster Drugs.J Bioeth Inq. 2021 Sep;18(3):477-497. doi: 10.1007/s11673-021-10119-x. Epub 2021 Sep 6. J Bioeth Inq. 2021. PMID: 34487285 Free PMC article. Review.
-
From the EBM pyramid to the Greek temple: a new conceptual approach to Guidelines as implementation tools in mental health.Epidemiol Psychiatr Sci. 2017 Apr;26(2):105-114. doi: 10.1017/S2045796016000767. Epidemiol Psychiatr Sci. 2017. PMID: 28290273 Free PMC article.
-
Financial Conflicts of Interest Among the Authors of the Clinical Practice Guidelines for Rheumatoid Arthritis in Japan.Cureus. 2023 Oct 7;15(10):e46650. doi: 10.7759/cureus.46650. eCollection 2023 Oct. Cureus. 2023. PMID: 37937008 Free PMC article.
-
Validity of studies suggesting preoperative chemotherapy for resectable thoracic esophageal cancer: A critical appraisal of randomized trials.World J Gastrointest Oncol. 2020 Jan 15;12(1):113-123. doi: 10.4251/wjgo.v12.i1.113. World J Gastrointest Oncol. 2020. PMID: 31966919 Free PMC article.
-
Financial Conflicts of Interest in Clinical Practice Guidelines: A Systematic Review.Mayo Clin Proc Innov Qual Outcomes. 2021 Jan 19;5(2):466-475. doi: 10.1016/j.mayocpiqo.2020.09.016. eCollection 2021 Apr. Mayo Clin Proc Innov Qual Outcomes. 2021. PMID: 33997642 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources