Laquinimod rescues striatal, cortical and white matter pathology and results in modest behavioural improvements in the YAC128 model of Huntington disease
- PMID: 27528441
- PMCID: PMC4985819
- DOI: 10.1038/srep31652
Laquinimod rescues striatal, cortical and white matter pathology and results in modest behavioural improvements in the YAC128 model of Huntington disease
Abstract
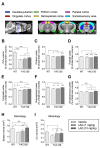
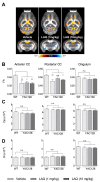
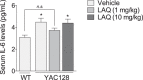
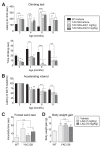
Increasing evidence supports a role for abnormal immune activation and inflammatory responses in Huntington disease (HD). In this study, we evaluated the therapeutic potential of laquinimod (1 and 10 mg/kg), a novel immunomodulatory agent shown to be protective in a number of neuroinflammatory conditions, in the YAC128 mouse model of HD. Treatment with laquinimod for 6 months rescued atrophy in the striatum, in certain cortical regions, and in the corpus callosum of YAC128 HD mice. Diffusion tensor imaging showed that white matter microstructural abnormalities in the posterior corpus callosum were improved following treatment with low dose (1 mg/kg) laquinimod, and were paralleled by reduced levels of interleukin-6 in the periphery of YAC128 HD mice. Functionally, treatment with laquinimod (1 and 10 mg/kg) led to modest improvements in motor function and in depressive-like behaviour. Taken together, these results suggest that laquinimod may improve some features of pathology in HD, and provides support for the role of immune activation in the pathogenesis of HD.
Conflict of interest statement
Yes, there is potential competing interest. L. H., S. P. and M. R. H. are employees of Teva Pharmaceuticals. Teva Pharmaceuticals played no role in the treatment or testing of animals, or the collection, analysis and interpretation of the results.
Figures
Similar articles
-
Laquinimod Treatment Improves Myelination Deficits at the Transcriptional and Ultrastructural Levels in the YAC128 Mouse Model of Huntington Disease.Mol Neurobiol. 2019 Jun;56(6):4464-4478. doi: 10.1007/s12035-018-1393-1. Epub 2018 Oct 17. Mol Neurobiol. 2019. PMID: 30334188
-
Early pridopidine treatment improves behavioral and transcriptional deficits in YAC128 Huntington disease mice.JCI Insight. 2017 Dec 7;2(23):e95665. doi: 10.1172/jci.insight.95665. JCI Insight. 2017. PMID: 29212949 Free PMC article.
-
Structural and molecular myelination deficits occur prior to neuronal loss in the YAC128 and BACHD models of Huntington disease.Hum Mol Genet. 2016 Jul 1;25(13):2621-2632. doi: 10.1093/hmg/ddw122. Epub 2016 Apr 28. Hum Mol Genet. 2016. PMID: 27126634 Free PMC article.
-
Laquinimod decreases Bax expression and reduces caspase-6 activation in neurons.Exp Neurol. 2016 Sep;283(Pt A):121-8. doi: 10.1016/j.expneurol.2016.06.008. Epub 2016 Jun 11. Exp Neurol. 2016. PMID: 27296315
-
Laquinimod treatment in the R6/2 mouse model.Sci Rep. 2017 Jul 10;7(1):4947. doi: 10.1038/s41598-017-04990-1. Sci Rep. 2017. PMID: 28694434 Free PMC article.
Cited by
-
C57BL/6 Background Attenuates mHTT Toxicity in the Striatum of YAC128 Mice.Int J Mol Sci. 2021 Nov 23;22(23):12664. doi: 10.3390/ijms222312664. Int J Mol Sci. 2021. PMID: 34884469 Free PMC article.
-
Promises and pitfalls of immune-based strategies for Huntington's disease.Neural Regen Res. 2017 Sep;12(9):1422-1425. doi: 10.4103/1673-5374.215245. Neural Regen Res. 2017. PMID: 29089980 Free PMC article. Review.
-
The contribution of preclinical magnetic resonance imaging and spectroscopy to Huntington's disease.Front Aging Neurosci. 2024 Feb 13;16:1306312. doi: 10.3389/fnagi.2024.1306312. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38414634 Free PMC article. Review.
-
Laquinimod Treatment Improves Myelination Deficits at the Transcriptional and Ultrastructural Levels in the YAC128 Mouse Model of Huntington Disease.Mol Neurobiol. 2019 Jun;56(6):4464-4478. doi: 10.1007/s12035-018-1393-1. Epub 2018 Oct 17. Mol Neurobiol. 2019. PMID: 30334188
-
Immunotherapies in Huntington's disease and α-Synucleinopathies.Front Immunol. 2020 Feb 25;11:337. doi: 10.3389/fimmu.2020.00337. eCollection 2020. Front Immunol. 2020. PMID: 32161599 Free PMC article. Review.
References
-
- Tabrizi S. J. et al.. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet neurology 12, 637–649 (2013). - PubMed
-
- Ross C. A. & Tabrizi S. J. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet neurology 10, 83–98 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

