LaeA regulation of secondary metabolism modulates virulence in Penicillium expansum and is mediated by sucrose
- PMID: 27528575
- PMCID: PMC6638289
- DOI: 10.1111/mpp.12469
LaeA regulation of secondary metabolism modulates virulence in Penicillium expansum and is mediated by sucrose
Abstract
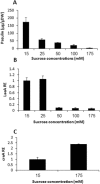
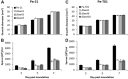
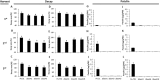
Penicillium expansum, the causal agent of blue mould rot, is a critical health concern because of the production of the mycotoxin patulin in colonized apple fruit tissue. Although patulin is produced by many Penicillium species, the factor(s) activating its biosynthesis are not clear. Sucrose, a key sugar component of apple fruit, was found to modulate patulin accumulation in a dose-responsive pattern. An increase in sucrose culture amendment from 15 to 175 mm decreased both patulin accumulation and expression of the global regulator laeA by 175- and five-fold, respectively, whilst increasing expression of the carbon catabolite repressor creA. LaeA was found to regulate several secondary metabolite genes, including the patulin gene cluster and concomitant patulin synthesis in vitro. Virulence studies of ΔlaeA mutants of two geographically distant P. expansum isolates (Pe-21 from Israel and Pe-T01 from China) showed differential reduction in disease severity in freshly harvested fruit, ranging from no reduction for Ch-Pe-T01 strains to 15%-25% reduction for both strains in mature fruit, with the ΔlaeA strains of Is-Pe-21 always showing a greater loss in virulence. The results suggest the importance of abiotic factors in LaeA regulation of patulin and other secondary metabolites that contribute to pathogenicity.
Keywords: Penicillium; mycotoxin; pathogenicity; patulin; sucrose.
© 2016 BSPP AND JOHN WILEY & SONS LTD.
Figures





Similar articles
-
Fungal attack and host defence pathways unveiled in near-avirulent interactions of Penicillium expansum creA mutants on apples.Mol Plant Pathol. 2018 Dec;19(12):2635-2650. doi: 10.1111/mpp.12734. Epub 2018 Oct 22. Mol Plant Pathol. 2018. PMID: 30047230 Free PMC article.
-
Ammonia activates pacC and patulin accumulation in an acidic environment during apple colonization by Penicillium expansum.Mol Plant Pathol. 2016 Jun;17(5):727-40. doi: 10.1111/mpp.12327. Epub 2015 Dec 3. Mol Plant Pathol. 2016. PMID: 26420024 Free PMC article.
-
The APSES factor PeStuA regulates the growth, conidiation, patulin production, and virulence of the postharvest fungus Penicillium expansum.Food Microbiol. 2025 Dec;132:104841. doi: 10.1016/j.fm.2025.104841. Epub 2025 Jun 17. Food Microbiol. 2025. PMID: 40683721
-
Penicillium expansum: biology, omics, and management tools for a global postharvest pathogen causing blue mould of pome fruit.Mol Plant Pathol. 2020 Nov;21(11):1391-1404. doi: 10.1111/mpp.12990. Epub 2020 Sep 23. Mol Plant Pathol. 2020. PMID: 32969130 Free PMC article. Review.
-
Molecular basis and regulation of pathogenicity and patulin biosynthesis in Penicillium expansum.Compr Rev Food Sci Food Saf. 2020 Nov;19(6):3416-3438. doi: 10.1111/1541-4337.12612. Epub 2020 Aug 28. Compr Rev Food Sci Food Saf. 2020. PMID: 33337032 Review.
Cited by
-
Patulin in Apples and Apple-Based Food Products: The Burdens and the Mitigation Strategies.Toxins (Basel). 2018 Nov 15;10(11):475. doi: 10.3390/toxins10110475. Toxins (Basel). 2018. PMID: 30445713 Free PMC article. Review.
-
Fungal attack and host defence pathways unveiled in near-avirulent interactions of Penicillium expansum creA mutants on apples.Mol Plant Pathol. 2018 Dec;19(12):2635-2650. doi: 10.1111/mpp.12734. Epub 2018 Oct 22. Mol Plant Pathol. 2018. PMID: 30047230 Free PMC article.
-
Modulation of extracellular Penicillium expansum-driven acidification by Papiliotrema terrestris affects biosynthesis of patulin and has a possible role in biocontrol activity.Front Microbiol. 2022 Aug 1;13:973670. doi: 10.3389/fmicb.2022.973670. eCollection 2022. Front Microbiol. 2022. PMID: 35979494 Free PMC article.
-
New Insight Into Pathogenicity and Secondary Metabolism of the Plant Pathogen Penicillium expansum Through Deletion of the Epigenetic Reader SntB.Front Microbiol. 2020 Apr 9;11:610. doi: 10.3389/fmicb.2020.00610. eCollection 2020. Front Microbiol. 2020. PMID: 32328048 Free PMC article.
-
Growth-Phase Sterigmatocystin Formation on Lactose Is Mediated via Low Specific Growth Rates in Aspergillus nidulans.Toxins (Basel). 2016 Nov 28;8(12):354. doi: 10.3390/toxins8120354. Toxins (Basel). 2016. PMID: 27916804 Free PMC article.
References
-
- Abdollahi, A. and Buchanan, R. (1981) Regulation of aflatoxin biosynthesis: characterization of glucose as an apparent inducer of aflatoxin production. J. Food Sci. 46, 143–146.
-
- Baert, K. , Devlieghere, F. , Flyps, H. , Oosterlinck, M. , Ahmed, M.M. , Rajković, A. , Verlinden, B. , Nicolaï, B. , Debevere, J. and De Meulenaer, B. (2007) Influence of storage conditions of apples on growth and patulin production by Penicillium expansum . Int. J. Food Microbiol. 119, 170–181. - PubMed
-
- Ballester, A.R. , Marcet‐Houben, M. , Levin, E. , Sela, N. , Selma‐Lazaro, C. , Carmona, L. , Wisniewski, M. , Droby, S. , Gonzalez‐Candelas, L. and Gabaldon, T. (2015) Genome, transcriptome, and functional analyses of Penicillium expansum provide new insights into secondary metabolism and pathogenicity. Mol. Plant–Microbe Interact. 28, 232–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

