Activated G Protein Gαs Samples Multiple Endomembrane Compartments
- PMID: 27528603
- PMCID: PMC5034030
- DOI: 10.1074/jbc.M116.729731
Activated G Protein Gαs Samples Multiple Endomembrane Compartments
Abstract
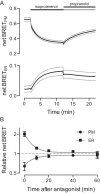
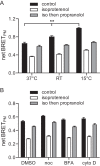
Heterotrimeric G proteins are localized to the plasma membrane where they transduce extracellular signals to intracellular effectors. G proteins also act at intracellular locations, and can translocate between cellular compartments. For example, Gαs can leave the plasma membrane and move to the cell interior after activation. However, the mechanism of Gαs translocation and its intracellular destination are not known. Here we use bioluminescence resonance energy transfer (BRET) to show that after activation, Gαs rapidly associates with the endoplasmic reticulum, mitochondria, and endosomes, consistent with indiscriminate sampling of intracellular membranes from the cytosol rather than transport via a specific vesicular pathway. The primary source of Gαs for endosomal compartments is constitutive endocytosis rather than activity-dependent internalization. Recycling of Gαs to the plasma membrane is complete 25 min after stimulation is discontinued. We also show that an acylation-deacylation cycle is important for the steady-state localization of Gαs at the plasma membrane, but our results do not support a role for deacylation in activity-dependent Gαs internalization.
Keywords: G protein-coupled receptor (GPCR); endosome; endosomes; heterotrimeric G protein; intracellular trafficking; lipase; protein acylation; protein palmitoylation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
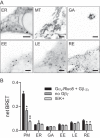
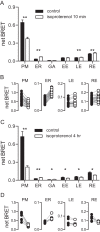
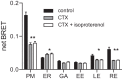
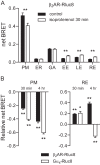

References
-
- Gilman A. G. (1987) G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56, 615–649 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

