BAR Domain-Containing FAM92 Proteins Interact with Chibby1 To Facilitate Ciliogenesis
- PMID: 27528616
- PMCID: PMC5064215
- DOI: 10.1128/MCB.00160-16
BAR Domain-Containing FAM92 Proteins Interact with Chibby1 To Facilitate Ciliogenesis
Abstract
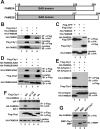
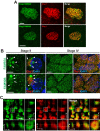
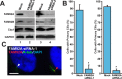
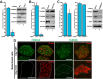
Chibby1 (Cby1) is a small, conserved coiled-coil protein that localizes to centrioles/basal bodies and plays a crucial role in the formation and function of cilia. During early stages of ciliogenesis, Cby1 is required for the efficient recruitment of small vesicles at the distal end of centrioles to facilitate basal body docking to the plasma membrane. Here, we identified family with sequence similarity 92, member A (FAM92A) and FAM92B, which harbor predicted lipid-binding BAR domains, as novel Cby1-interacting partners using tandem affinity purification and mass spectrometry. We found that in cultured cell lines, FAM92A colocalizes with Cby1 at the centrioles/basal bodies of primary cilia, while FAM92B is undetectable. In airway multiciliated cells, both FAM92A and -92B colocalize with Cby1 at the base of cilia. Notably, the centriolar localization of FAM92A and -92B depends largely on Cby1. Knockdown of FAM92A in RPE1 cells impairs ciliogenesis. Consistent with the membrane-remodeling properties of BAR domains, FAM92A and -92B in cooperation with Cby1 induce deformed membrane-like structures containing the small GTPase Rab8 in cultured cells. Our results therefore suggest that FAM92 proteins interact with Cby1 to promote ciliogenesis via regulation of membrane-remodeling processes.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Conditional knockout mice for the distal appendage protein CEP164 reveal its essential roles in airway multiciliated cell differentiation.PLoS Genet. 2017 Dec 15;13(12):e1007128. doi: 10.1371/journal.pgen.1007128. eCollection 2017 Dec. PLoS Genet. 2017. PMID: 29244804 Free PMC article.
-
Dimerization of the BAR domain-containing protein FAM92A modulates lipid binding and interaction with CBY1.J Biol Chem. 2025 Jul;301(7):110346. doi: 10.1016/j.jbc.2025.110346. Epub 2025 Jun 6. J Biol Chem. 2025. PMID: 40484380 Free PMC article.
-
Cby1 promotes Ahi1 recruitment to a ring-shaped domain at the centriole-cilium interface and facilitates proper cilium formation and function.Mol Biol Cell. 2014 Oct 1;25(19):2919-33. doi: 10.1091/mbc.E14-02-0735. Epub 2014 Aug 7. Mol Biol Cell. 2014. PMID: 25103236 Free PMC article.
-
Role of DZIP1-CBY-FAM92 transition zone complex in the basal body to membrane attachment and ciliary budding.Biochem Soc Trans. 2020 Jun 30;48(3):1067-1075. doi: 10.1042/BST20191007. Biochem Soc Trans. 2020. PMID: 32491167 Review.
-
BARMR1/FAM92A1, a novel gene encoding BAR domain protein with multi-functions.Gene. 2021 Jan 10;765:145074. doi: 10.1016/j.gene.2020.145074. Epub 2020 Sep 3. Gene. 2021. PMID: 32891772 Review.
Cited by
-
Cep131-Cep162 and Cby-Fam92 complexes cooperatively maintain Cep290 at the basal body and contribute to ciliogenesis initiation.PLoS Biol. 2024 Mar 5;22(3):e3002330. doi: 10.1371/journal.pbio.3002330. eCollection 2024 Mar. PLoS Biol. 2024. PMID: 38442096 Free PMC article.
-
Membrane remodeling by FAM92A1 during brain development regulates neuronal morphology, synaptic function, and cognition.Nat Commun. 2024 Jul 23;15(1):6209. doi: 10.1038/s41467-024-50565-w. Nat Commun. 2024. PMID: 39043703 Free PMC article.
-
The human ciliopathy protein RSG1 links the CPLANE complex to transition zone architecture.Nat Commun. 2025 Jul 1;16(1):5701. doi: 10.1038/s41467-025-61005-8. Nat Commun. 2025. PMID: 40593758 Free PMC article.
-
nNOS regulates ciliated cell polarity, ciliary beat frequency, and directional flow in mouse trachea.Life Sci Alliance. 2021 Mar 2;4(5):e202000981. doi: 10.26508/lsa.202000981. Print 2021 May. Life Sci Alliance. 2021. PMID: 33653689 Free PMC article.
-
DZIP1 regulates mammalian cardiac valve development through a Cby1-β-catenin mechanism.Dev Dyn. 2021 Oct;250(10):1432-1449. doi: 10.1002/dvdy.342. Epub 2021 Apr 9. Dev Dyn. 2021. PMID: 33811421 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
