Cell autonomous regulation of herpes and influenza virus infection by the circadian clock
- PMID: 27528682
- PMCID: PMC5018795
- DOI: 10.1073/pnas.1601895113
Cell autonomous regulation of herpes and influenza virus infection by the circadian clock
Abstract
Viruses are intracellular pathogens that hijack host cell machinery and resources to replicate. Rather than being constant, host physiology is rhythmic, undergoing circadian (∼24 h) oscillations in many virus-relevant pathways, but whether daily rhythms impact on viral replication is unknown. We find that the time of day of host infection regulates virus progression in live mice and individual cells. Furthermore, we demonstrate that herpes and influenza A virus infections are enhanced when host circadian rhythms are abolished by disrupting the key clock gene transcription factor Bmal1. Intracellular trafficking, biosynthetic processes, protein synthesis, and chromatin assembly all contribute to circadian regulation of virus infection. Moreover, herpesviruses differentially target components of the molecular circadian clockwork. Our work demonstrates that viruses exploit the clockwork for their own gain and that the clock represents a novel target for modulating viral replication that extends beyond any single family of these ubiquitous pathogens.
Keywords: circadian; clock; herpes; influenza; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
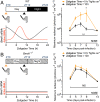

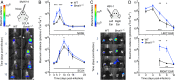

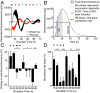
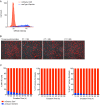

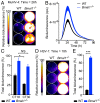
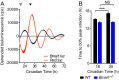

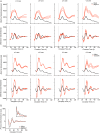



References
-
- van der Horst GT, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398(6728):627–630. - PubMed
-
- O’Neill JS, Maywood ES, Hastings MH. Cellular mechanisms of circadian pacemaking: Beyond transcriptional loops. Handbook Exp Pharmacol. 2013;(217):67–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

