Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters
- PMID: 27528686
- PMCID: PMC5024618
- DOI: 10.1073/pnas.1519555113
Rapid hyperosmotic-induced Ca2+ responses in Arabidopsis thaliana exhibit sensory potentiation and involvement of plastidial KEA transporters
Abstract
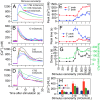
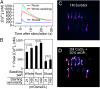
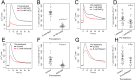
Plants experience hyperosmotic stress when faced with saline soils and possibly with drought stress, but it is currently unclear how plant roots perceive this stress in an environment of dynamic water availabilities. Hyperosmotic stress induces a rapid rise in intracellular Ca(2+) concentrations ([Ca(2+)]i) in plants, and this Ca(2+) response may reflect the activities of osmo-sensory components. Here, we find in the reference plant Arabidopsis thaliana that the rapid hyperosmotic-induced Ca(2+) response exhibited enhanced response magnitudes after preexposure to an intermediate hyperosmotic stress. We term this phenomenon "osmo-sensory potentiation." The initial sensing and potentiation occurred in intact plants as well as in roots. Having established a quantitative understanding of wild-type responses, we investigated effects of pharmacological inhibitors and candidate channel/transporter mutants. Quintuple mechano-sensitive channels of small conductance-like (MSL) plasma membrane-targeted channel mutants as well as double mid1-complementing activity (MCA) channel mutants did not affect the response. Interestingly, however, double mutations in the plastid K(+) exchange antiporter (KEA) transporters kea1kea2 and a single mutation that does not visibly affect chloroplast structure, kea3, impaired the rapid hyperosmotic-induced Ca(2+) responses. These mutations did not significantly affect sensory potentiation of the response. These findings suggest that plastids may play an important role in early steps mediating the response to hyperosmotic stimuli. Together, these findings demonstrate that the plant osmo-sensory components necessary to generate rapid osmotic-induced Ca(2+) responses remain responsive under varying osmolarities, endowing plants with the ability to perceive the dynamic intensities of water limitation imposed by osmotic stress.
Keywords: abscisic acid; calcium; osmotic sensing; plastid; salt stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Hu H, Xiong L. Genetic engineering and breeding of drought-resistant crops. Annu Rev Plant Biol. 2014;65(1):715–741. - PubMed
-
- Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA. Plant drought stress: Effects, mechanisms and management. In: Lichtfouse E, Navarrete M, Debaeke P, Véronique S, Alberola C, editors. Sustainable Agriculture. Springer Netherlands; Dordrecht, The Netherlands: 2009. pp. 153–188.
-
- Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12(5):1067–1078. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

