Safety, Antitumor Activity, and Immune Activation of Pegylated Recombinant Human Interleukin-10 (AM0010) in Patients With Advanced Solid Tumors
- PMID: 27528724
- PMCID: PMC5657013
- DOI: 10.1200/JCO.2016.68.1106
Safety, Antitumor Activity, and Immune Activation of Pegylated Recombinant Human Interleukin-10 (AM0010) in Patients With Advanced Solid Tumors
Abstract
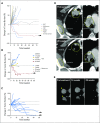
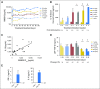
Purpose Interleukin-10 (IL-10) stimulates the expansion and cytotoxicity of tumor-infiltrating CD8+ T cells and inhibits inflammatory CD4+ T cells. Pegylation prolongs the serum concentration of IL-10 without changing the immunologic profile. This phase I study sought to determine the safety and antitumor activity of AM0010. Patients and Methods Patients with selected advanced solid tumors were treated with AM0010 in a dose-escalation study, which was followed by a renal cell cancer (RCC) dose-expansion cohort. AM0010 was self-administered subcutaneously at doses of 1 to 40 μg/kg once per day. Primary end points were safety and tolerability; clinical activity and immune activation were secondary end points. Results In the dose-escalation and -expansion cohorts, 33 and 18 patients, respectively, were treated with daily subcutaneous injection of AM0010. AM0010 was tolerated in a heavily pretreated patient population. Treatment-related adverse events (AEs) included anemia, fatigue, thrombocytopenia, fever, and injection site reactions. Grade 3 to 4 nonhematopoietic treatment-related AEs, including rash (n = 2) and transaminitis (n = 1), were observed in five of 33 patients. Grade 3 to 4 anemia or thrombocytopenia was observed in five patients. Most treatment-related AEs were transient or reversible. AM0010 led to systemic immune activation with elevated immune-stimulatory cytokines and reduced transforming growth factor beta in the serum. Partial responses were observed in one patient with uveal melanoma and four of 15 evaluable patients with RCC treated at 20 μg/kg (overall response rate, 27%). Prolonged stable disease of at least 4 months was observed in four patients, including one with colorectal cancer with disease stabilization for 20 months. Conclusion AM0010 has an acceptable toxicity profile with early evidence of antitumor activity, particularly in RCC. These data support the further evaluation of AM0010 both alone and in combination with other immune therapies and chemotherapies.
Trial registration: ClinicalTrials.gov NCT02009449.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Interleukin-10: An Immune-Activating Cytokine in Cancer Immunotherapy.J Clin Oncol. 2016 Oct 10;34(29):3576-3578. doi: 10.1200/JCO.2016.69.6435. J Clin Oncol. 2016. PMID: 27573656 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

