The aerodynamics and control of free flight manoeuvres in Drosophila
- PMID: 27528778
- PMCID: PMC4992712
- DOI: 10.1098/rstb.2015.0388
The aerodynamics and control of free flight manoeuvres in Drosophila
Abstract
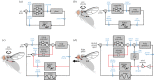
A firm understanding of how fruit flies hover has emerged over the past two decades, and recent work has focused on the aerodynamic, biomechanical and neurobiological mechanisms that enable them to manoeuvre and resist perturbations. In this review, we describe how flies manipulate wing movement to control their body motion during active manoeuvres, and how these actions are regulated by sensory feedback. We also discuss how the application of control theory is providing new insight into the logic and structure of the circuitry that underlies flight stability.This article is part of the themed issue 'Moving in a moving medium: new perspectives on flight'.
Keywords: Drosophila; aerodynamics; control theory; flight; insects.
© 2016 The Author(s).
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

