Preference for pharmaceutical formulation and treatment process attributes
- PMID: 27528802
- PMCID: PMC4970633
- DOI: 10.2147/PPA.S101821
Preference for pharmaceutical formulation and treatment process attributes
Abstract
Purpose: Pharmaceutical formulation and treatment process attributes, such as dose frequency and route of administration, can have an impact on quality of life, treatment adherence, and disease outcomes. The aim of this literature review was to examine studies on preferences for pharmaceutical treatment process attributes, focusing on research in diabetes, oncology, osteoporosis, and autoimmune disorders.
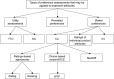
Methods: The literature search focused on identifying studies reporting preferences for attributes of the pharmaceutical treatment process. Studies were required to use formal quantitative preference assessment methods, such as utility valuation, conjoint analysis, or contingent valuation. Searches were conducted using Medline, EMBASE, Cochrane Library, Health Economic Evaluation Database, and National Health Service Economic Evaluation Database (January 1993-October 2013).
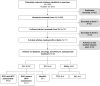
Results: A total of 42 studies met inclusion criteria: 19 diabetes, nine oncology, five osteoporosis, and nine autoimmune. Across these conditions, treatments associated with shorter treatment duration, less frequent administration, greater flexibility, and less invasive routes of administration were preferred over more burdensome or complex treatments. While efficacy and safety often had greater relative importance than treatment process, treatment process also had a quantifiable impact on preference. In some instances, particularly in diabetes and autoimmune disorders, treatment process attributes had greater relative importance than some or all efficacy and safety attributes. Some studies suggested that relative importance of treatment process depends on disease (eg, acute vs chronic) and patient (eg, injection experience) characteristics.
Conclusion: Despite heterogeneity in study methods and design, some general patterns of preference clearly emerged. Overall, the results of this review suggest that treatment process has a quantifiable impact on preference and willingness to pay for treatment, even in many situations where safety and efficacy were the primary concerns. Patient preferences for treatment process attributes can inform drug development decisions to better meet the needs of patients and deliver improved outcomes.
Keywords: conjoint; contingent valuation; pharmaceutical formulation; preference; treatment process; utility.
Figures
References
-
- Hixson-Wallace JA, Dotson JB, Blakey SA. Effect of regimen complexity on patient satisfaction and compliance with warfarin therapy. Clin Appl Thromb Hemost. 2001;7(1):33–37. - PubMed
-
- Morris LS, Schulz RM. Medication compliance: the patient’s perspective. Clin Ther. 1993;15(3):593–606. - PubMed
-
- Shikiar R, Rentz A, Barone J, Duncanson F, Katz E. Patient satisfaction with ofloxacin (F) and polymyxin B/neomycin/hydrocortisone (C) in the treatment of otitis externa: results from two randomized clinical trials. J Manage Care Med. 2002;6(3):24–27.
-
- Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7(2):204–215. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

