Comparison of Two Synthetic Bone Graft Products in a Rabbit Posterolateral Fusion Model
- PMID: 27528855
- PMCID: PMC4910791
Comparison of Two Synthetic Bone Graft Products in a Rabbit Posterolateral Fusion Model
Abstract
Background: The drawbacks of iliac crest autograft as graft material for spine fusion are well reported. Despite continued modifications to improve bone healing capacity, the efficacy of synthetic graft materials as stand-alone replacements remains uncertain. The rabbit posterolateral fusion model is an established environment for testing of fusion concepts. It offers the opportunity to obtain radiographic, biomechanical and histological data on novel fusion materials. The objective of this study was to compare the spine fusion capability of two synthetic bone graft products in an established rabbit posterolateral spine fusion (PLF) model: Signafuse® Bioactive Bone Graft Putty and Actifuse® ABX.
Methods: Bilateral intertransverse spine fusion was performed at the L5-L6 transverse processes (TPs) of New Zealand White rabbits using either Signafuse or Actifuse ABX as the bone graft material. Bone remodeling and spine fusion were assessed at 6 and 12 weeks using radiographic, biomechanical and histological endpoints.
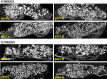
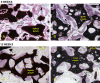
Results: Fusion rate by manual palpation at 6 weeks was greater for Signafuse (33%) compared to Actifuse ABX (0%), and equivalent in both groups at 12 weeks (50%). Biomechanical fusion rate based on flexion-extension data was 80% in Signafuse group and 44% for Actifuse ABX. Histology revealed a normal healing response in both groups. MicroCT and histomorphometric data at 6 weeks showed greater new bone formation in the Signafuse group compared to Actifuse ABX (p <0.05), with no differences detected at 12 weeks. Histological fusion scores were greater in the Signafuse group at 6 and 12 weeks, indicated by higher degree structural remodeling and tendency towards complete bridging of the fusion bed compared to the Actifuse ABX group.
Conclusion: Confirmed by several metrics, Signafuse outperformed Actifuse ABX as a standalone synthetic bone graft in an established PLF model, demonstrating greater rates of bone remodeling and spine fusion. The combination of 45S5 bioactive glass and biphasic HA/βTCP granules of Signafuse appear to provide greater bone healing capability in comparison to the 0.8% silicate-substituted hydroxyapatite material of Actifuse ABX.
Figures


Similar articles
-
Electrospun PLGA and β-TCP (Rebossis-85) in a Lapine Posterolateral Fusion Model.Iowa Orthop J. 2019;39(2):9-19. Iowa Orthop J. 2019. PMID: 32577102 Free PMC article.
-
Influence of 45S5 Bioactive Glass in A Standard Calcium Phosphate Collagen Bone Graft Substitute on the Posterolateral Fusion of Rabbit Spine.Iowa Orthop J. 2017;37:193-198. Iowa Orthop J. 2017. PMID: 28852357 Free PMC article.
-
Assessment of SiCaP-30 in a Rabbit Posterolateral Fusion Model with Concurrent Chemotherapy.Iowa Orthop J. 2015;35:140-6. Iowa Orthop J. 2015. PMID: 26361457 Free PMC article.
-
Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel.Spine (Phila Pa 1976). 1998 Jan 15;23(2):159-67. doi: 10.1097/00007632-199801150-00003. Spine (Phila Pa 1976). 1998. PMID: 9474720 Review.
-
Factors influencing arthrodesis rates in a rabbit posterolateral spine model with iliac crest autograft.Eur Spine J. 2014 Feb;23(2):426-34. doi: 10.1007/s00586-013-3074-0. Epub 2013 Oct 29. Eur Spine J. 2014. PMID: 24166021 Free PMC article. Review.
Cited by
-
Effects of systemically administered abaloparatide, an osteoanabolic PTHrP analog, as an adjuvant therapy for spinal fusion in rats.JOR Spine. 2020 Nov 25;4(1):e1132. doi: 10.1002/jsp2.1132. eCollection 2021 Mar. JOR Spine. 2020. PMID: 33778406 Free PMC article.
-
Efficacy of a synthetic calcium phosphate with submicron surface topography as autograft extender in lapine posterolateral spinal fusion.J Biomed Mater Res B Appl Biomater. 2019 Aug;107(6):2080-2090. doi: 10.1002/jbm.b.34301. Epub 2019 Jan 7. J Biomed Mater Res B Appl Biomater. 2019. PMID: 30614621 Free PMC article.
-
High posterior cervical fusion rates with iliac autograft and Nanoss/bone marrow aspirate.Surg Neurol Int. 2017 Jul 20;8:152. doi: 10.4103/sni.sni_241_17. eCollection 2017. Surg Neurol Int. 2017. PMID: 28808601 Free PMC article.
-
Electrospun PLGA and β-TCP (Rebossis-85) in a Lapine Posterolateral Fusion Model.Iowa Orthop J. 2019;39(2):9-19. Iowa Orthop J. 2019. PMID: 32577102 Free PMC article.
-
Synthetic osteobiologics in spine surgery: a review.Turk J Med Sci. 2024 Oct 25;55(1):43-51. doi: 10.55730/1300-0144.5941. eCollection 2025. Turk J Med Sci. 2024. PMID: 40104322 Free PMC article. Review.
References
-
- Arrington ED, Smith WJ, Chambers HG, et al. Complications of iliac crest bone graft harvesting. Clin.Orthop.Relat Res. 1996:300–9. - PubMed
-
- Ebraheim NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J.Am.Acad.Orthop.Surg. 2001;9:210–8. - PubMed
-
- Hu R, Hearn T, Yang J. Bone graft harvest site as a determinant of iliac crest strength. Clin.Orthop. Relat Res. 1995:252–6. - PubMed
-
- Hu RW, Bohlman HH. Fracture at the iliac bone graft harvest site after fusion of the spine. Clin.Orthop.Relat Res. 1994:208–13. - PubMed
-
- Kahn B. Superior gluteal artery laceration, a complication of iliac bone graft surgery. Clin.Orthop.Relat Res. 1979:204–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
