The Potential Role of Kallistatin in the Development of Abdominal Aortic Aneurysm
- PMID: 27529213
- PMCID: PMC5000709
- DOI: 10.3390/ijms17081312
The Potential Role of Kallistatin in the Development of Abdominal Aortic Aneurysm
Abstract
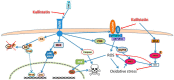
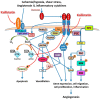
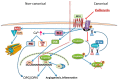
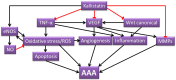
Abdominal aortic aneurysm (AAA) is a vascular condition that causes permanent dilation of the abdominal aorta, which can lead to death due to aortic rupture. The only treatment for AAA is surgical repair, and there is no current drug treatment for AAA. Aortic inflammation, vascular smooth muscle cell apoptosis, angiogenesis, oxidative stress and vascular remodeling are implicated in AAA pathogenesis. Kallistatin is a serine proteinase inhibitor, which has been shown to have a variety of functions, potentially relevant in AAA pathogenesis. Kallistatin has been reported to have inhibitory effects on tumor necrosis factor alpha (TNF-α) signaling induced oxidative stress and apoptosis. Kallistatin also inhibits vascular endothelial growth factor (VEGF) and Wnt canonical signaling, which promote inflammation, angiogenesis, and vascular remodeling in various pre-clinical experimental models. This review explores the potential protective role of kallistatin in AAA pathogenesis.
Keywords: abdominal aortic aneurysm; kallistatin; oxidative stress; serine proteinase inhibitors; vascular remodelling.
Figures
Similar articles
-
Kallistatin limits abdominal aortic aneurysm by attenuating generation of reactive oxygen species and apoptosis.Sci Rep. 2021 Aug 31;11(1):17451. doi: 10.1038/s41598-021-97042-8. Sci Rep. 2021. PMID: 34465809 Free PMC article. Clinical Trial.
-
Kallistatin: double-edged role in angiogenesis, apoptosis and oxidative stress.Biol Chem. 2017 Nov 27;398(12):1309-1317. doi: 10.1515/hsz-2017-0180. Biol Chem. 2017. PMID: 28742513 Review.
-
Role of vascular endothelial growth factor-A in development of abdominal aortic aneurysm.Cardiovasc Res. 2011 Jul 15;91(2):358-67. doi: 10.1093/cvr/cvr080. Epub 2011 Mar 24. Cardiovasc Res. 2011. PMID: 21436157
-
Tumor necrosis factor-α converting enzyme is a key mediator of abdominal aortic aneurysm development.Atherosclerosis. 2011 Oct;218(2):470-8. doi: 10.1016/j.atherosclerosis.2011.06.008. Epub 2011 Jun 13. Atherosclerosis. 2011. PMID: 21722904
-
Protective Role of Endogenous Kallistatin in Vascular Injury and Senescence by Inhibiting Oxidative Stress and Inflammation.Oxid Med Cell Longev. 2018 Dec 2;2018:4138560. doi: 10.1155/2018/4138560. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30622668 Free PMC article. Review.
Cited by
-
Meprin-α (Mep1A) enhances TNF-α secretion by mast cells and aggravates abdominal aortic aneurysms.Br J Pharmacol. 2020 Jun;177(12):2872-2885. doi: 10.1111/bph.15019. Epub 2020 Mar 17. Br J Pharmacol. 2020. PMID: 32072633 Free PMC article.
-
The SERPINA4 rs2070777 AA Genotype is Associated with an Increased Risk of Recurrent Miscarriage in a Southern Chinese Population.Int J Womens Health. 2021 Jan 18;13:111-117. doi: 10.2147/IJWH.S290009. eCollection 2021. Int J Womens Health. 2021. PMID: 33500667 Free PMC article.
-
Angiogenesis in Aortic Aneurysm and Dissection: A Literature Review.Rev Cardiovasc Med. 2023 Aug 1;24(8):223. doi: 10.31083/j.rcm2408223. eCollection 2023 Aug. Rev Cardiovasc Med. 2023. PMID: 39076698 Free PMC article. Review.
-
Is Abdominal Aortic Aneurysm Behavior after Endovascular Repair Associated with Aneurysm Wall Density on Computed Tomography Angiography?Medicina (Kaunas). 2019 Jul 25;55(8):406. doi: 10.3390/medicina55080406. Medicina (Kaunas). 2019. PMID: 31349723 Free PMC article.
-
The mechanism and therapy of aortic aneurysms.Signal Transduct Target Ther. 2023 Feb 3;8(1):55. doi: 10.1038/s41392-023-01325-7. Signal Transduct Target Ther. 2023. PMID: 36737432 Free PMC article. Review.
References
-
- Kniemeyer H.W., Kessler T., Reber P.U., Ris H.B., Hakki H., Widmer M.K. Treatment of ruptured abdominal aortic aneurysm, a permanent challenge or a waste of resources? Prediction of outcome using a multi-organ-dysfunction score. Eur. J. Vasc. Endovasc. Surg. 2000;19:190–196. doi: 10.1053/ejvs.1999.0980. - DOI - PubMed
-
- The UK small aneurysm trial participants Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649–1655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

